Research
1.
Metal speciation
a.
Biogeochemistry of trace metals in the natural environment
b. Trace metal
speciation and bioavailability in natural waters
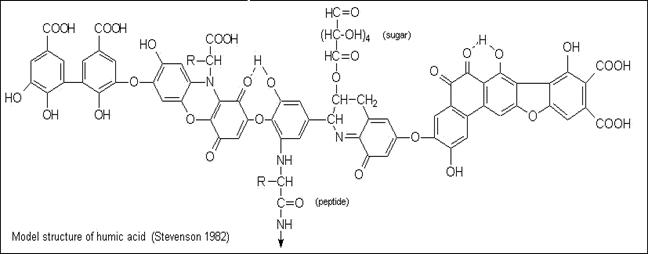
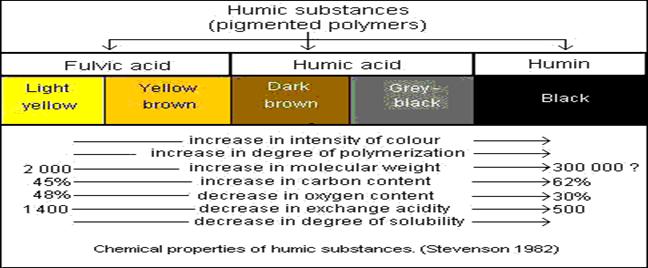
In natural waters, trace metals exist in
many different physical and chemical forms (such as
free metal ions, metals complexed with naturally
occurring ligands such as humic substances or inorganic ions such as
NTA, EDTA, and metals bound to solids) which determine
their mobility, bioavailability, and toxicity to aquatic organisms.
Therefore, the determination of relevant species of trace metals, in
addition to their total metal concentrations, will be of utmost
importance in improving our understanding of the processes and the
biogeochemical pathways that govern their fate and ultimate impact
on the environment.
2.
Characterization and analysis of airborne particulate matter
Particulate Matter (PM) is an
air pollutant consisting of a mixture of solid and liquid particles
suspended in the air. Different names are being used for types or
fractions of particulate matter, defined either by particle size or
by sampling method. The most commonly used names include: TSP (total
suspended particulates), PM10, PM2.5, coarse particles, fine
particles, ultrafine particles, BS (black smoke) and BC (black
carbon). In general, smaller particles (PM10 and smaller) are more
important for health effects than larger particles since they
penetrate deeper into the lungs.

Micro-Orifice Uniform
Deposition Impactors (MOUDI) for precision, high accuracy aerosol
sampling and collecting size-fractionated particle samples for
gravimetric and/or chemical analysis.
Micro-Orifice Uniform Deposition
Impactors (MOUDI).
3.
Isotope fractionations
The general idea is indeed that
all processes which involve chemical or physical reactions result in
a fractionation of isotopes. So natural variations could be observed
in environment samples after physical processes like evaporation or
after biogeochemical processes such as methylation for example. So
isotopic ratios could be used to trace in environmental samples, the
source of metal and elucidate his biogeochemical behavior.
Isotope fractionation is the physical phenomenon which causes
changes in the relative abundance of isotopes due to their
differences in mass. There are two categories of isotope effects:
equilibrium and kinetic.
An equilibrium isotope effect will cause one isotope to
concentrate in one component of a reversible system that is in
equilibrium. If it is theta heavier isotope that concentrates in the
component of interest, then that component is commonly referred to
as enriched or heavy. If it is the light isotope that concentrates
then the component is referred to as depleted or light. In most
circumstances the heavy isotope concentrates in the component in
which the element is bound more strongly and thus equilibrium
isotope effects usually reflect relative differences in the bond
strengths of the isotopes in the various components of the system.
A kinetic isotope effect occurs when one isotope reacts
more rapidly than the other in an irreversible system or a system in
which the products are swept away from the reactants before they
have an opportunity to come to equilibrium. Normally, the lighter
isotope will react more rapidly than the heavy isotope and thus the
product will be lighter than the reactant.
4.
Analytical chemistry
Development of analytical techniques for chemical
speciation of trace metals in the natural environment.
a-
Inductively-Coupled Plasma - Mass Spectrometry (ICP-MS)
b-
Electrochemical techniques (ASV-RDE and AdCSV)