| 4. Forms of Corrosion | |
|
4.10 Caustic Cracking |
|
Caustic Cracking
It is an intercrystalline cracking of boiler carbon steel caused by a corrosion reaction along the grain boundaries within the metal as shown in the figure below. Leaking drum seam in riveted drum boilers are vulnerable to caustic embrittlement.
Factors Leading to Caustic Cracking
High hydrate alkalinity
High tensile stresses
Causes of Caustic Cracking
Caustic is a boiler water additive. It is added to preserve the thin film of iron oxide to protect the boiler from corrosion. The following are the causes of concentration of caustics:
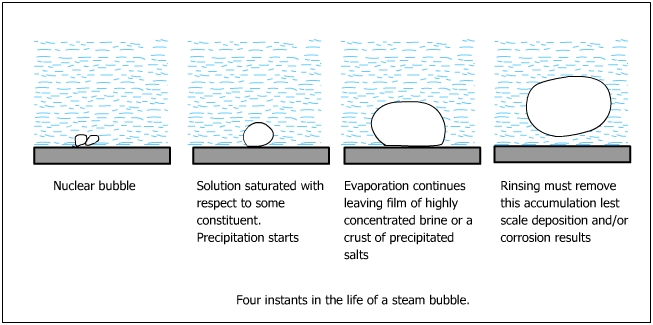
Small bubbles of steam nucleated at the metal surface in minute concentrations of solids in the boiler water would be deposited on the metal surface. As the solids are formed they are simultaneously removed from the metal surface by water which re-dissolves them. However, when the rate of bubble formation exceeds the rate of dissolution of the solids, concentration of caustics would begin to increase.
The deposits of solids shield the metal from the back water. Steam forms under the deposits and escapes leaving behind a caustic residue.
Caustic also concentrates by evaporation if a water line exists.
Mechanism
Concentrated caustic dissolves the protective magnetite oxides
4NaOH
+ Fe3O4 ![]() 2NaFeO2 + Na2FeO2 + 2H2O
2NaFeO2 + Na2FeO2 + 2H2O
As soon as magnetite (Fe3O4) is destroyed water reacts directly with iron as
3Fe + 4H2O
![]() Fe3O4 + 4H2
Fe3O4 + 4H2
Also caustic may react with iron to form hydrogen
Fe + NaOH ![]() NaFeO2 + H2
NaFeO2 + H2
As discussed earlier, if hydrogen is released in the atomic form, it diffuses into the steel. Some of the diffused atomic hydrogen will combine at metal grain boundaries or voids or inclusions to form H2 (molecular hydrogen) as discussed earlier in hydrogen cracking. It may also react with iron carbide (Fe3C) to produce methane
Fe3C
+ 4H ![]() CH4 + 3Fe
CH4 + 3Fe
The accumulation of hydrogen or methane would create internal pressures which on exceeding the tensile strength of the metal would cause the separation of the metal at the grain boundaries. It may also lead to the bursting of the boiler tubes.
More examples of caustic cracking are given in figures below.
| Excessive NaOH in boiler water leads to tube metal gouging as shown in this sample from a boiler tube. | |
| Caustic gouging also occurs due to evaporation along a waterline without significant accumulation of deposit as shown in the cross section of a boiler tube. |
Prevention
Addition of inhibitors suppress caustic embrittlement. Sodium nitrite is added in correct ratio with sodium hydroxide to suppress caustic embrittlement.
Prevent excessive water side deposits.
Prevent creation of water lines in tubes.
Prevent contamination of steam.
Reduce the amount of sodium hydroxide additives.
|
|