| 4. Forms of Corrosion | |
|
4.8 Hydrogen Induced Cracking |
|
Hydrogen Induced Cracking (HIC)
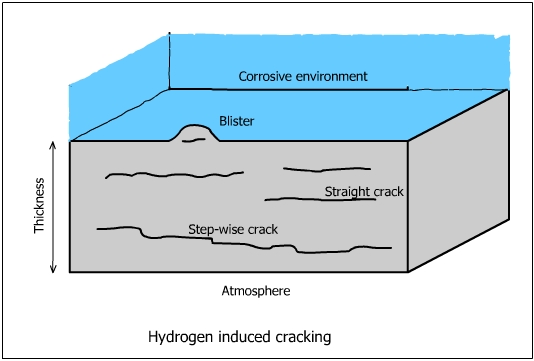
The mechanism of hydrogen induced cracking is not well understood. It is basically the same as that of hydrogen blistering. It occurs generally in low strength steels used for pipings, tanks and vessels.
Mechanism
Basically, the mechanism depends on the diffusion of atomic hydrogen into voids. The atomic hydrogen combines to form molecular hydrogen and forms rows of small blisters (cracks). The pressure inside the blisters continues to build up as more H atoms combine to form hydrogen molecules. Blisters are formed at varying depths which link together to form cracks. The cracks formed by bulging of the blisters are parallel to the surface along the original lamination generate in various depths and finally connected together. The cracks may reach the surface and the metal fails.
MnS inclusions are highly susceptible to Hydrogen Induced Cracking. HIC is predominantly observed in killed steels (Addition of Si and aluminum to keep low level of oxygen for a C-O reaction during solidification of the ingot). Steels in which teh sulfide inclusions are in a globular form are less susceptible.
The mechanism may be summarized in the following steps
Source of atomic
hydrogen H2S ![]() 2H+ + S2-
2H+ + S2-
Diffusion
2Ho ![]() H2
H2
Accumulation in the void
Linking of voids to form cracks.
Given below is a typical example of hydrogen induced cracking in an oil pipeline.
Another example of hydrogen damage in boiler tubes and its corresponding microstructure. (Source)
Prevention
Reduce corrosion rates. For instance, addition of inhibitors can be used to suppress hydrogen evolution.
Baking steels at low temperature (100 - 150 C)
Use coatings on linings (alloys, cement ceramic)
Use copper and cobalt alloyed steels
|
|