
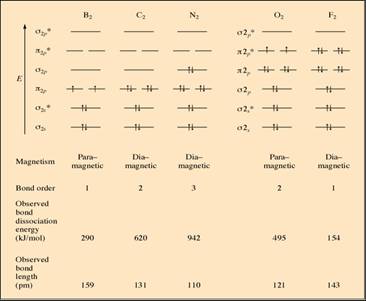
Figure 9.39
Molecular Orbital Summary of Second Row Diatomic
Molecular Orbital Summary of Second Row Diatomic

Ø“B.O. increase, energy for bonds
increases, and bond length decreases”
ØB2-bond energy > F2-bond
energy. Although both has B.O. = 1, this can be related to greater e-e repulsion in F2 than B2
ØThe very strong bond in N2 is the main reason that many nitrogen
containing compounds are high explosive
ØO2-molecules are known to be
paramagnetic because of its MO-electronic configuration.