
Valence
Bond Theory and NH3
N – 1s22s22p3
3 H – 1s1
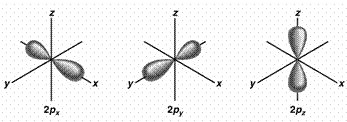
If use the
3 2p orbitals
predict 900
Actual H-N-H
bond angle is
107.30

If the bonds form from overlap of 3 2p
orbitals on nitrogen
with the 1s orbital on each hydrogen atom,
what would
the molecular geometry of NH3 be?