15.4 Measuring Pressure
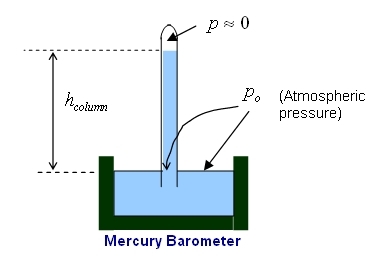
A barometer is a device that uses a liquid in a tube to measure the atmospheric pressure. A glass tube is filled with the liquid and inverted with its open end in a dish of the same liquid. The liquid that is used in real barometers is mercury (Hg), however we will be discussing a "Water Barometer" physlet (see the illustration on the right) to explain the principle of operation.
 |
| Script by Dr. Mohamed S. Kariapper using Physlets from |
|
There are two pressure sensors (small red rectangles, one in the middle and one on the left) in the physlet which can be moved to show the pressure at a given point. Move these pressure sensors to measure the pressures at various points. You will find (using the middle sensor) that the pressure at point V (interface between the 'vacuum' and the water), is equal to zero. And as you move the sensor down, the pressure at points A , B and C increases linearly with depth according to the formula we derived in the last section for fluid at rest:
where is the depth below the point V.
Within the same (continuous) liquid, the
pressure is the same at the same depth (level). Check the pressures
at
When mercury is used as the liquid we have the real Barometer and for mercury is 760 mm (76.0 cm). Therefore the atmospheric pressure is given as 760 torr (1 torr = 1 mm Hg (millimeter of mercury)).
Open-tube Manometer
The open tube manometer measures the gauge pressure of a gas. The device is shown in the figure below.

|
||||||||||||||||||||||||||||||||||||