A heat engine operates between 600 K and 300 K. In each cycle
it takes 100 J from the hot reservoir, loses 25 J to the cold
reservoir, and does 75 J of work. This heat engine violates:
a The second law but not the first law of thermodynamics.
b The first law but not the second law of the thermodynamics.
c Both, the first law and the second law of thermodynamics.
d Conservation of energy.
e Neither the first law nor the second law.
==============================================
Question 2
Five moles of an ideal monatomic gas are taken though the cycle
shown in the Figure (1). Calculate the efficiency of the cycle.
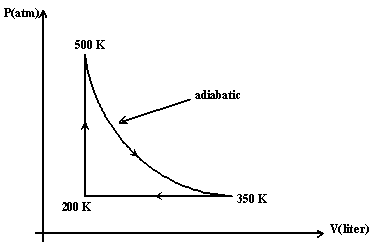
a 0.17.
b 0.06.
c 0.28.
d 0.45.
e 0.83.
==============================================
Question 3
A 4.0-kg piece of iron at 800 K is dropped into a lake
whose temperature is 280 K. Assume that the lake is so
large that its temperature rise is negligible.
Find the change in the entropy of the lake.
[specific heat of iron = 0.11 kcal/kg.K].
a zero
b + 0.20 kcal/K
c + 0.82 kcal/K
d - 0.82 kcal/K
e - 0.20 kcal/K
==============================================
Question 4
50.0 g of water at 15.0 degrees-C are converted slowly
into ice at - 15.0 degrees-C.
What is the change of entropy of water ?
specific heat of water = 4190 J/kg.K.
specific heat of ice = 2220 J/kg.K.
Lf = 333kJ/kg, Lv = 2256kJ/kg
a + 78.5 J/K
b - 78.5 J/K
c + 83.4 J/K
d - 17.5 J/K
e - 83.4 J/K
==============================================
Question 5
A Carnot refrigerator has a coefficient of performance
equal to 5. The refrigerator absorbs 120 J of heat from a
cold reservoir in each cycle.
How much heat is expelled to the hot reservoir ?
a 600 J
b 480 J
c 144 J
d 720 J
e 125 J
==============================================
Question 6
A Carnot heat engine operates between two reservoirs
whose temperatures are 27 degrees-C and 127 degrees-C.
If we want to double the efficiency of the heat engine,
what should be the temperature of the hot reservoir?
Assume the temperature of the cold reservoir is kept
constant.
a 800 K
b 600 K
c 1500 K
d 1200 K
e 900 K
==============================================
Question 7
A sample of an ideal monatomic gas undergoes
the reversible process A to B displayed in the
T-S diagram shown in figure 6. The process is :
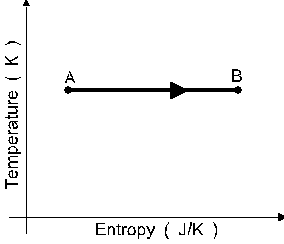
a an isothermal compression.
b a change of phase.
c a free expansion.
d a constant-volume process.
e an isothermal expansion.
==============================================
Question 8
Five moles of an ideal gas undergo a reversible isothermal
compression from volume V to volume V/2 at temperature
30 degrees C. What is the change in the entropy of the gas?
a -18 J/K.
b 18 J/K.
c -29 J/K.
d 29 J/K.
e -81 J/K.
==============================================
Question 9
An automobile engine operates with an overall efficiency
of 20%. How many gallons of gasoline is wasted for each
10 gallons burned?
a 6.
b 12.
c 10.
d 2.
e 8.
==============================================
Question 10
A Carnot heat engine absorbs 70.0 kJ as heat and expels
55.0 kJ as heat in each cycle. If the low-temperature
reservoir is at 120 degrees-C, find the temperature of
the high-temperature reservoir.
a 35.8 degrees-C
b 153 degrees-C
c 227 degrees-C
d 450 degrees-C
e 393 degrees-C
==============================================
Question 11
One mole of an ideal gas is taken through the reversible
cycle shown in figure 1, with Q1 = 6.0 kJ, Q2 = 30 kJ,
Q3 = 18 kJ and Q4 = 10 kJ.
What is the efficiency of this cycle ?
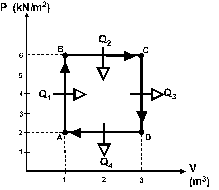
a 0.54
b 0.29
c 0.33
d 0.22
e 0.44
==============================================
Question 12
240 grams of water at 8 degrees-C are cooled to ice at
at - 5 degrees-C. Calculate the change in entropy of the
water.
c(ice)=2090 J/kg*K, c(water)=4186 J/kg*K, Lf=3.33*10**(5) J/Kg.
a 254 J/K.
b -172 J/K.
c -254 J/K.
d -331 J/K.
e 331 J/K.
==============================================
Question 13
Which one of the following statements is WRONG?
a A refrigerator works like a heat engine in reverse.
b No heat engine has higher efficiency than Carnot efficiency.
c Thermal energy cannot be transferred spontaneously from a
cold object to a hot object.
d After a system has gone through a reversible cyclic process,
its total entropy does not change.
e The total entropy of a system increases only if it absorbs
heat.
==============================================
Answers
1 a
2 a
3 c
4 b
5 c
6 b
7 e
8 c
9 e
10 c
11 d
12 d
13 e