| Objective |
Demonstration of Galvanic Corrosion. |
|
| Materials |
The following galvanic couples were prepared |
|
|
Plate and Rivette Assemblies
|
 |
|
|
|
||
|
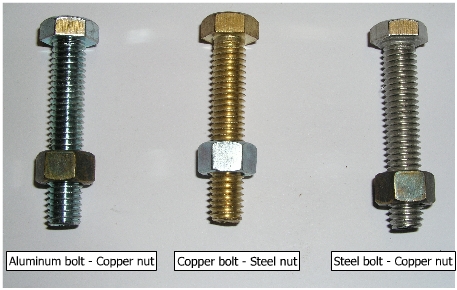
Nut and bolt assembly |
||
|
(a) Steel nut and copper bolt |
||
|
(b) Copper bolt and steel nut |
||
|
(c) Aluminum bolt with copper nut |
||
|
|
||
|
Electrolyte: |
||
|
All assemblies were immersed in a 3.5 wt% NaCl solution |
||
|
|
||
| Observations |
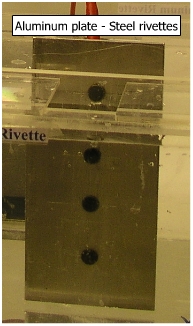
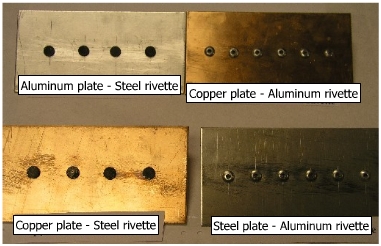
(a) Coupling of steel and aluminum In galvanic series Aluminum is anodic to Steel (-1.03 V vs -0.44 V). Aluminum plate has a larger anodic area and steel rivettes, smaller cathodic area. Because of the larger anodic area, the current density would be very small, and then corrosion is hardly expected. Corrosion on aluminum plate is observed as shown in adjacent figure.. |
|
|
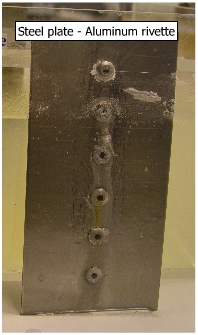
An opposite situation is shown in adjacent figure where steel plate is joined to aluminum rivette. The corrosion is clearly shown on aluminum rivettes because of smaller anodic area. |
|
|
|
(b) Copper coupled to Steel In this case, copper plate is cathodic to steel (+0.344 V) and has a larger area, where as steel rivettes are anodic to copper (-0.44 V) and has a smaller area. The steel rivetees are subjected to a higher anodic current density because of a smaller area and hence, subjected to corrosion. Corrosion of steel rivettes can be clearly observed in the picture. |
|
|
|
When steel plates are coupled with copper rivettes, the situation here is reversed to what is shown above because of larger surface area of steel plate which are anodic to copper, the steel plates are not corroded. The pair is a good example of minimizing corrosion by keeping a larger anodic area and a smaller cathodic area. |
|
|
|
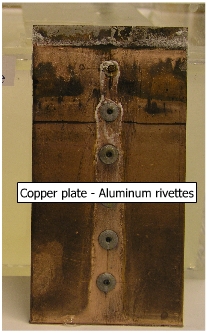
A copper plate with Aluminum rivette is shown in this figure. It is a case of large cathodic (copper) and small anodic (aluminum) area, resulting in corrosion of aluminum rivette. |
|
|
|
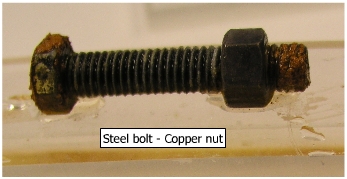
Nut and Bolt Assemblies: (a)
Steel bolt and copper
nut assembly Steel bolt is anodic to copper nut and has a larger surface area. Copper nut is cathodic to steel. Hence there is no appreciable evidence of corrosion in the assembly.
The reverse situation is shown below. Corrosion is clearly observed on steel nut, because of smaller anodic area.
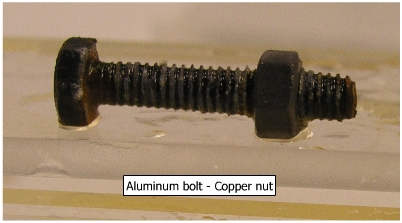
(b)
Aluminum bolt and copper
nut Because of larger anodic area of aluminum bolt and a smaller cathodic area of copper nut, corrosion is not appreciable in this system.
|
||
|
|
||
|
|
||
|
|
||