| Objective | To demonstrate the method of measurement of corrosion potential for some metals. |
| Materials | High impedance Voltmeter |
| Polarization cell | |
|
Specimens of Aluminum plate or rod, Copper plate or rod, Brass plate or
rod. |
|
| Reference Electrode ( standard calomel electrode) | |
| 3.5% Na Cl solution | |
|
|
|
| Procedure |
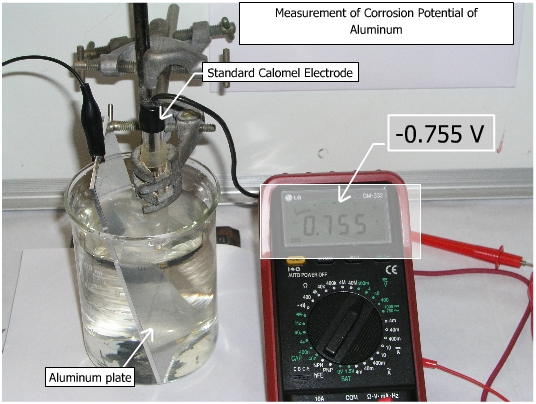
Corrosion potential is defined as the potential of a corroding metal in a particular environment. This potential denotes whether the metal is active or in a passive state. Connect the positive lead of the voltmeter to the reference electrode (SCE) and negative lead to the specimen (working electrode). Set the voltmeter to ON position and record the reading. The reading obtained is the corrosion potential of the specimen with respect to standard calomel electrode.
|
|
|
|

|
|
|
|
|
| Conclusion |
The method of measurement of corrosion potential against standard calomel electrode has been demonstrated for different metals. |