| 4. Forms of Corrosion | |
|
4.11 Crevice Corrosion [1/2] |
|
Crevice Corrosion
It is a form of localized corrosion which occurs inside the crevices formed on a metal surface. Oxygen is consumed preferentially inside the crevice in preference to the bulk solution. It commonly occurs where water is stagnant due to the design of a component. Crevice corrosion may also be caused by formation of built of concentration cells, such as differential aeration cells.
Factors Leading to Crevice Corrosion
Presence of narrow spaces between metal to metal or non-metal to metal components
Presence of cracks, cavities and other physical defects
Deposition of barnacles, biofouling and other deposits
Deposits of dirt, mud and other deposits.
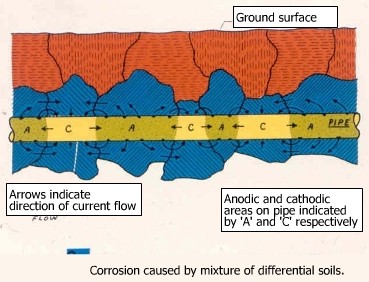
Developing of Differential Aeration Cell
A differential oxygen concentration cell is formed when two areas of an electrolyte contain different concentrations of oxygen. For instance, soil on the upper surface may contain more oxygen than the soil underneath the pipe, and may form a differential aeration cell. The soil enriched in oxygen forming the cathode and that deficient in oxygen forming the anode.
Given below are some examples of Crevice corrosion.
| Formation of a concentration cell in the presence of high and low oxygen rich soils. | |
| Crevice corrosion in a typical 316 Stainless Steel pipe after exposure to an acid environment. Crevice attack occurred where two metal surfaces were held in contact by bolts in flange. Note that the external surfaces where crevice was not tight were not significantly attacked. | |
| Crevice corrosion under a rubber band in contact with stainless steel in a solution of NaCl and FeCl3. | |
| Crevice corrosion in a gear assembly | |
| A ball bearing showing cold fusion and crevice corrosion. | |
| A nut and bolt assembly showing crevice corrosion in cold rolled threads. The corrosion products are accumulated at the junction of the nut and the bolt. |
|
|