| 4. Forms of Corrosion | |
|
4.2 Uniform Corrosion [2/2] |
|
Factors Affecting Uniform Corrosion
1. Hot and humid environment: The rate of corrosion is higher in hot and humid areas like Eastern Province in Saudi Arabia, Kualalumpur, Singapore, Bangkok and Karachi because of hot weather, high humidity and persistently wet conditions. Corrosion is not promoted by cold and dry climatic conditions. It would not be expected in places like Alaska in North America with an extremely cold climate or in Abha in Saudi Arabia with very dry condition.
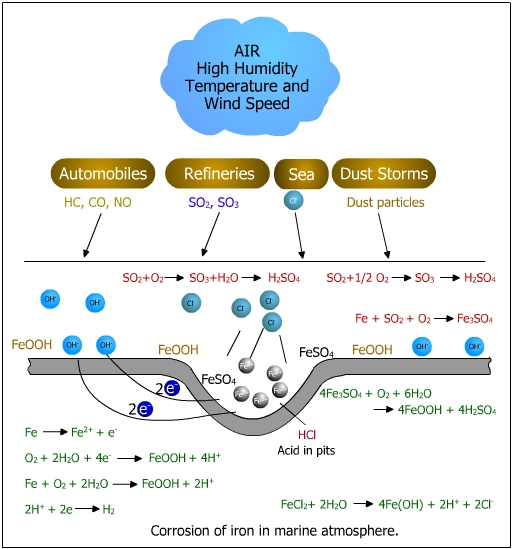
2. Environmental contamination: Corrosion is accelerated by environmental pollution caused by emission from automobiles, refineries, petrochemical and chemical industries. The presence of silica particles (SiO2), SO2 particles and CO2 and marine salts. In the presence of impurities, corrosion can proceed in as little as 35% relative humidity. The effect of environmental contaminants on uniform corrosion is shown in the figure below.
Mechanism of Uniform Corrosion:
At the anode, we have Fe
![]() Fe2+
+ 2e
Fe2+
+ 2e
At the cathode, O2
+ 4H+ + 4e ![]() 2H2O and O2 +
4H+ + 4e
2H2O and O2 +
4H+ + 4e ![]() 2H2O
2H2O
Rust is formed by the interaction of
positively charged Fe2+ ions with the negatively charged OH-
ions as Fe2+ + 2OH- ![]() Fe(OH)2 which is insoluble and separates from the
electrolyte. A more familiar name for Fe(OH)2 is rust (a white
green precipitate). With more access to oxygen, Fe(OH)2 oxidizes to ferric
hydroxide Fe(OH)3 which in turn converts to Fe2O3;H2O.
Fe(OH)2 which is insoluble and separates from the
electrolyte. A more familiar name for Fe(OH)2 is rust (a white
green precipitate). With more access to oxygen, Fe(OH)2 oxidizes to ferric
hydroxide Fe(OH)3 which in turn converts to Fe2O3;H2O.
Different types of rust such as Fe3O4 (black magnetite), g-Fe3O4 (brown rust) g(FeOOH) (yellow rust) are observed depending on the nature of the interaction in the environment. The mechanism of rust formation is shown in the figure below.
Examples:
| 1. Tarnishing of silverware. |
| 2. Corrosion of offshore drilling platforms. |
| 3. Corrosion of automobile bodies. |
| 4. Rusting metallic structures in historical monuments. |
| 5. Rusting of electric poles. |
| 6. Rusting of decks and bridges. |
Prevention:
1. Give proper corrosion allowance at the designing stage. For instance, increase the wall thickness in proportion to the expected decrease in thickness in a specified time.
2. Coat the metal surface: Select a primer compatible with the environment. For instance Zinc primer is most suitable for painting of underground structures.
3. Use cathodic protection, if justified.
4. Use regional experience for selection of materials.
|
|