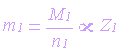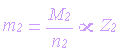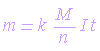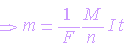The classical
electrochemical work conducted by Michael Faraday in the Nineteenth century
produced two laws published in 1833 and 1834 named after him. The two laws can
be summarized below.
First Law
The mass
of primary products formed at an electrode by electrolysis is directly
proportional to the quantity of electricity passed. Thus:
|
m  I t
I t |
|
m = Z I t |
| where |
| I =
current in amperes |
| t =
time in seconds |
| m = mass of the primary product |
| Z = constant
of proportionality (electrochemical equivalent). It is the mass of a
substance liberated by 1 ampere-second of a current (1 coulomb) |
Second Law
The masses of different primary products
formed by equal amounts of electricity are proportional to the ratio of molar
mass to the number of electrons involved with a particular reaction:
|
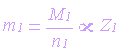
|
|
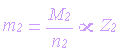
|
| where |
| m1, m2 =
masses of primary product |
| M1, M2 =
molar masses (g/mol) |
| n1, n2 = number
of electrons |
| Z1, Z2 =
electrochemical equivalent |
combining the First Law and
the Second Law, we get
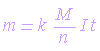
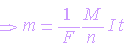
where F
= Faraday’s constant. It is the quantity of electricity required to deposit
the ratio of mass to valency of any substance and expressed in coulombs per
mole (C.mol-1). It has a value of 96,485
couloumbs per mole.