| 2. Understanding Corrosion | |
|
2.9 Resistance Polarization |
Resistance (Ohmic) Polarization
It is known that current is transported from the anode to the cathode by ions in the electrolyte and in the metallic path from the anode to cathode. Because of their normal high conductivity, metals offer little resistance to the current flow in the metallic path. However, resistance can be encountered if the distance between the anode and cathode is appreciable.
The problem of the ohmic polarization may be significant where this current flows from the anode to the cathode in an electrolyte, depending on the resistance of the electrolyte.
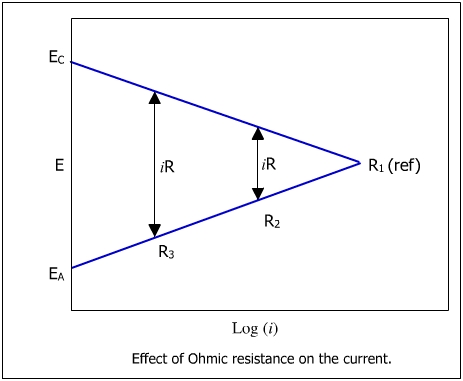
The figure below shows the effect of ohmic resistance on the current.
Consider an electrolyte such as seawater with a low resistivity or high conductivity represented by R. In the seawater as electrolyte, the anodic and cathodic polarization curve would intersect at the point R, where the potentials of the anode and the cathode are polarized at the same value.
If the resistivity of the solution is high, a potential drop (iR) would result from the flow of the current, the resistivity and the potential of the anode and cathode would differ.
The reason is that the potential drop (iR) diminishes the driving force of activation polarization reaction and the anodic and cathodic reactions are not polarized to the same potentials. The situation is represented by R2 in the figure. On further increasing the electrolyte resistance, the magnitude of the ohmic drop would further increase as shown by R3 in the figure. With increasing the resistance offered by the electrolytes, the magnitude of the corrosion current would decrease as shown by R1, R2 and R3 in the figure.
Interestingly, increasing the solution resistance offers a good method of controlling corrosion as decreasing corrosive current means decreasing corrosion.