| 2. Understanding Corrosion | |
|
2.6 A Look at Polarization [3/3] |
Points to Remember:
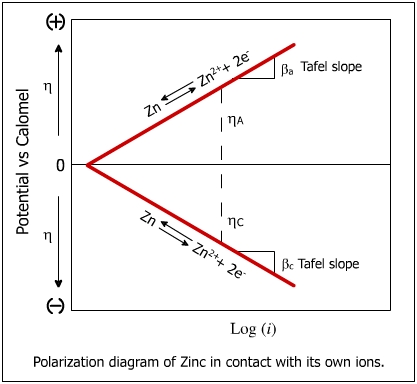
The potential of the polarized cathode is smaller than the potential of reversible electrode (h = 0). .The figure below shows the polarization of zinc.
hc < heq
The potential of the polarized anode is greater than the potential of reversible electrode
ha > heq
hcell > heq
FACTORS AFFECTING POLARIZATION
Current: Polarization will increase with increasing current
Area: The larger the area, the smaller would be the polarization
Temperature: The higher the temperature, the lower is the polarization
Agitation: The higher the agitation, the higher is the polarization
Concentration: For cathodic reactions involving dilute electrolytes polarize rapidly. In anodic reactions, polarization is the highest with concentrated electrolyte.