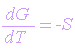
| 2. Understanding Corrosion | |
|
2.5.1 Relationship between 'Free Energy' and 'Equilibrium Constant' |
|
The contribution made by one mole of any constituent, A, to the total free energy, G, of the mixture is GA, which may be represented by
|
GA = GAo RT ln(aA) |
| where aA = activity of the substance, T = absolute temperature, and R = gas constant. |
From the Gibbs-Helmholtz equation:
G = H - TS
Substituting H from the First Law of Thermodynamics
G = U + PV - TS
Differentiating:
dG = dV + PdV + VdP - TdS - SdT
But,
dV = qrev - PdV and qrev = TdS
So,
dG = TdS - PdV + PdV + VdP - TdS - SdT
i.e., dG = VdP - SdT
At constant pressure, this equation becomes:
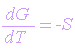
and at constant temperature:
dG = VdP
However, for ideal gas equation for 1 mole of reaction is
| pV = RT |
| V = RT/p |
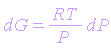 |
Integration between the limits gives
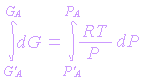
If G'A is taken to refer for standard conditions, it becomes GAo.
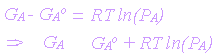 (1)
(1)
If activities are concerned instead of pressure, then
![]()
Applying DG for the reaction:
aA
+ bB ![]() cC
+ dD gives
cC
+ dD gives
![]()
Expressing GC, GD, GA and GB as shown in (1) above, we get
![]()
So, for 1 mole forward reaction,
![]() (2)
(2)
and at equilibrium, DG = 0
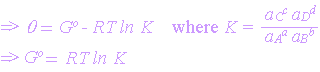
Equation (2) may be written as
![]()
The equations linking DG and K are referred to as VanHoff's Isotherms.
|
|