Since iron and steel are important constructional materials, it is important to understand how they corrode in an aqueous media. Consider only one anode and one cathode for simplicity in a piece of iron immersed in aerated water as shown in the following figure.
The anodic reaction would be Fe![]() Fe2+ + 2e
Fe2+ + 2e
The loss of electrons leaves positively charged ions at the anode which travel from the anode to cathode via the water and carry the positive current. The electrons which are released on the anode travel from the anode to the cathode via the metallic circuit. These electrons are utilized in the reduction of oxygen present in water which is in contact with the cathode. Hence, at the cathode, on of the following reactions may take place.
(1) O2 + 4H+
+ 4e![]() 2H2O (if the water is acidic)
2H2O (if the water is acidic)
(2) O2 + 2H2O
+ 4e![]() 4H+ (if the water is
basic)
4H+ (if the water is
basic)
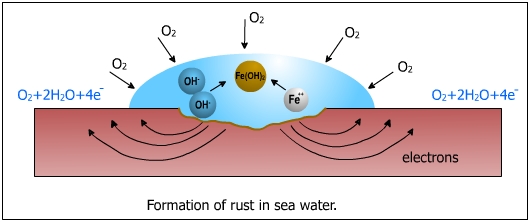
The negatively charged OH- ions react with the positively charged Fe2+ ions and forms Fe(OH)2 .
4Fe2+ +
2OH- ![]() 4Fe(OH)2 (white)
4Fe(OH)2 (white)
OR
4Fe(OH)2 + O2
+ 2H2O ![]() 2Fe2O3 (brown rust)
2Fe2O3 (brown rust)
The end product is Fe2O3, which is generally called "rust". The rust is formed a little away from the surface. The mechanism of rust formation is shown in the following figure.
Different colors of rust may be observed. The following reactions represent the corrosion of iron based materials.
-
FeO + H2
 FeO
+ 2H+ + 2e
FeO
+ 2H+ + 2e -
FeO + 2H2O
 Fe(OH)2 + 2H+ + 2e
Fe(OH)2 + 2H+ + 2e -
FeO + H2O
 Fe3O4 + 2H+ + 2e (black
magnetite)
Fe3O4 + 2H+ + 2e (black
magnetite) -
Fe3O4 + H2O
 3 g-Fe2O3 + 2H+
+ 2e (brown)
3 g-Fe2O3 + 2H+
+ 2e (brown) -
3 g-Fe2O3 + 3H2O
 6 g-FeOOH (yellow ferric oxide
hydrated)
6 g-FeOOH (yellow ferric oxide
hydrated)
As the corrosion produced layers are insulators, the iron is protected as long as it is covered by a thin uniform layer of oxides, however, as soon as the oxide layer is damaged, corrosion is accelerated.