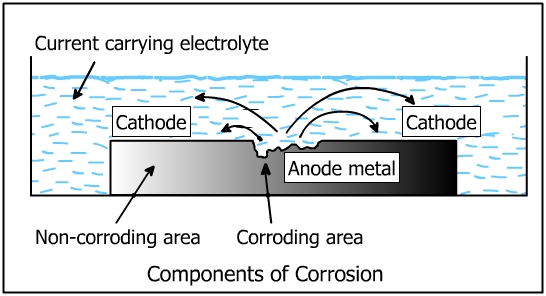
- Anode
- Cathode
- Electrolyte
- Metallic path
Fe![]() Fe2+
+ 2e
Fe2+
+ 2e
Al![]() Al3+
+ 3e
Al3+
+ 3e
Zn![]() Zn2+
+ 2e
Zn2+
+ 2e
Mg![]() Mg2+
+ 2e
Mg2+
+ 2e
(2) Cathode: It is the site where reduction takes place. The electrons released at the anode travel to the cathode by a metallic path where they react with the ions in the electrolyte and cause reduction of the positive ions. The cathodic reaction is accompanied by a gain of electrons. Following are the major cathodic reactinos in corrosion of metals in aqueous solution.
2H+ + e ![]() H2
H2
O2 + 4H+ + 4e ![]() 2H2O (in acid
solution)
2H2O (in acid
solution)
2H2O + O2 + 4e ![]() 4OH- (in neutral solution)
4OH- (in neutral solution)
(3) Electrolyte: It provides a conductive medium for passage of ions which act as charge carriers, like Fe2+ ions, which carry a positive charge and flow from anode to cathode.
(4) Metallic path: It provides the flow of electrons from the anode to cathode. All metals provide the electron path.
All the above four components are shown in the figure below. The flow of electric current is also shown.