A mixture of 1.00 mol H
2, and 1.00 mol I
2 were placed in a 2.00-L container and the reaction
2 HI (g)
⇌ H
2 (g)
+
I
2 (g)
was allowed to reach equilibrium at 430
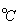
. If the equilibrium constant is 1.842 x 10
-2 , calculate the amount of HI present at equilibrium.