A piece of wood from a sunken ship is found to have a activity of
13 disintegrations min
–
1 g
–
1 while a freshly cut piece of wood is
found to have 15 disintegrations min
–
1 g
–
1. Approximately, how
long ago was this ship built given that the half-life of
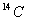
is 5760 years?