 doi:10.1016/S0013-7952(98)00044-1
doi:10.1016/S0013-7952(98)00044-1
Copyright © 1998 Elsevier Science B.V. All rights
reserved
Expansive characteristics of gypsiferous/anhydritic soil formations
This article is not included in your organization's
subscription. However, you may be able to
access
this article under your organization's agreement with Elsevier.
Shahid Azama,
Sahel N. Abduljauwadb,
*,
Naser A. Al-Shayeab
and Omar S. B. Al-Amoudib
a Department of Projects and Maintenance, KFUPM,
Dhahran, Saudi Arabia
b Department of Civil Engineering, KFUPM,
Dhahran, Saudi Arabia
Received 14 January 1998;
accepted 5 August 1998.
Available online 6 January 1999.
Abstract
Geology and climatic and environmental conditions have led to the formation
of expansive soils in the Eastern Province of Saudi Arabia. Calcium sulphate,
which commonly occurs in such soils, is well known for phase transformation and
dissolution. Phase changes from gypsum to anhydrite and vice versa, and
dissolution of these phases, add to the potential hazards of local expansive
soils. This paper discusses the behaviour of the expansive soil formations of
eastern Saudi Arabia containing gypsum and anhydrite.
Author Keywords: Calcium sulphate; Dissolution; Expansive
soil; Phase transformation
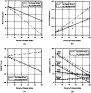
Fig. 1. Geotechnical properties of clay–calcium sulphate mixtures (a) water
content, (b) dry unit weight, (c) specific gravity and (d) Atterberg limits.
Fig. 2. Thermal analysis of gypsum.
Fig. 3. Thermal analysis of expansive clay.
Fig. 4. XRD results for calcium sulphate (a) gypsum, (b) anhydrite, (c)
bassanite and (d) expansive clay (S, smectite; I, illite; P, palygorskite; Q,
quartz).
Fig. 5. Percent swell of expansive clay and calcium sulphate phases.
Fig. 6. Micro-structural assessment of expansive clay: (a) SEM micrograph,
split mode with magnification ×1000 (left), ×6000 (right) and (b) EDX
spectrum.
Fig. 7. Micro-structural assessment of gypsum: (a) SEM micrograph, split mode
with magnification ×1000 (left), ×6000 (right) and (b) EDX spectrum.
Fig. 8. SEM micrograph of anhydrite, split mode with magnification ×1000
(left), ×6000 (right): (a) before and (b) after the inundation with water in the
swell test.
Fig. 9. Swell pressure of expansive clay and calcium sulphate phases.
Fig. 10. Percent swell of various synthetic clay–calcium sulphate
mixtures.
Fig. 11. SEM micrograph, split mode with magnification ×1000 (left), ×6000
(right), of clay–gypsum mixtures containing (a) 20% and (b) 80% gypsum.
Fig. 12. SEM micrograph, split mode with magnification ×1000 (left), ×6000
(right), of clay–anhydrite mixtures containing 20% anhydrite (a) before and (b)
after inundation with water in the swell test.
Fig. 13. SEM micrograph, split mode with magnification ×1000 (left), ×6000
(right), of clay–anhydrite mixtures containing 80% anhydrite (a) before and (b)
after inundation with water in the swell test.
Fig. 14. Void ratio–pressure test results for clay–anhydrite mixture
containing 50% anhydrite.
Fig. 15. Flow-time data for clay–anhydrite mixture containing 50%
anhydrite.
Fig. 16. Chemical analysis of percolating water through a clay–anhydrite
mixture containing 50% anhydrite.
Fig. 17. SEM micrograph, magnification ×500, of clay–anhydrite mixtures
containing 50% anhydrite leached with (a) water and (b) brine.
Table 1. Geotechnical properties of various clay–gypsum and clay–anhydrite
mixtures
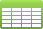
Table 2. Mineralogical composition of calcium sulphate phases

*Corresponding
author. Fax: +966 3 860 2879; e-mail: sajauwad@kfupm.edu.sa

.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)