|
|
|
NUCLEAR PROPERTIES
- Interaction between 50 nucleons is 50 !
- mass, radius, density, abundance, decay modes, half lives, reaction modes, cross sections, spin, mag. dipole moment, electric quadrupole moment, excited states.....
- Static Properties : charge, radius, mass ..........
- Dynamic Properties : decay, reaction
- 108 nuclides ( >1000 if we consider isotopes )
- Where to find properties of nuclides?!
(Journals, compiled sources (tables))
  THE NUCLEAR RADIUS
- The nucleus is approximately spherical Þ nuclear radius.
- 2 parameters : 1. Radius 2. Skin thickness
- 59Co was studied using neutrons (neutron scattering).
- Why is this diffraction pattern?
Density is changing in the nucleus so we have a diffraction pattern similar to light going through media of different index of refraction n.
- Why the diffraction pattern is not so pronounced?! (no sharp surface).
Nuclear density and nuclear potential have the same spatial dependence.
The Distribution of Nuclear Charge
- Radiation Scattering
- Beams of electrons with energies 100 MeV to 1 GeV are used.
- Diffraction patterns are analyzed and
- Initial wave
function
- Electron can be considered as a free particle
- After scattering of the electron
- The potential is
- Transition
probability
=
-
-
Substituting and integrating over r we get we get the normalized result -
-
for
elastic scattering
- F(q) = form factor
- Notice that the density is almost constant for all nuclei.
- # of nucleons/unit volume
- t is distance from
90% of
- Root mean square radius < r2 > =
- See other ways of calculating nuclear charge radius in Krane.
-
r1 = density at small values of r
Re = r at r = r1 / 2
t = surface thickness
- Charge density & matter density.
- It is found that nuclear matter density is the same for all nuclei aside from surface effects.
- t
- Re
- nuclear potential radius
R
THE DISTRIBUTION OF NUCLEAR MATTER
- Interaction between two nuclei due to nuclear force
- Consider the scattering of
- Particles should have enough energy to overcome the Coulomb
potential
- Another method of determining the nuclear
radius is
-
-
- Comparison with measured values
- The charge and matter radius of nuclei are nearly equal to within about 0.1f.
NUCLEAR MASS
- Nucleus has 99.97% of the mass of an atom.
- Old scale (O16) new scale (C12)
C12 = 12.000000 u
Neutron = 1.008665 u
Proton = 1.007277 u
What happened to the rest of the mass for a nucleus like
MASS AND ABUNDANCE OF NUCLIDES
-
Although we need to use nuclear masses in nuclear
reactions and decays, tables list the values of neutral atoms only
- The Binding Energy in the nuclear is large.
-
For a typical nucleus B.E
(for atomic B.E
- For quarks the B.E is about 0.99 of the total energy, so 3 quarks of total energy of ~ 300 Ge combine to produce a nucleon of rest energy 1 GeV.
- So it is not possible to separate the discussion of rest mass from binding energy.
- Experimentally how do we measure nuclear masses?
- The old and precise method is through Nuclear Mass Spectrometry.
- Neighboring isotopes differ in mass by approximately 1%.
- Mass Spectroscopes can measure masses to a precision of 10-6.
- A Mass Spectrograph has:
Ion Source Velocity Selector Momentum Selector electromagnetic field uniform magnetic field
qE = qvB will pass
Fr= FB
- For accuracy we measure the difference between two nearly equal masses (mass doublets). NUCLIDE ABUNDANCES - We can use the mass spectrometer to find the nuclide abundance also by varying E or B (see Fig. 3.14) SEPARATED ISOTOPES - If we set the Mass Spectrometer on a certain mass, we can produce large quantities of it to do experiments. Oak Ridge Nat. Lab sell these isotopes. LASER ISOTOPE SEPARATION - Set one laser to excite only certain atoms (How).A second laser will ionize these atoms only and can be collected using an electric field (Fig. 3.15). BINDING ENERGY
Or
What happened to the electron mass in (1) and B of electrons
Neutron Separation Energy
Proton Separation Energy Sp
Þ Similar to atomic ionization energies
- If we plot A vs B/A we notice the following
1. The curve is relatively constant except for light nuclei.
2. B/A
3. At A = 60 nuclei are most tight
hence we gain energy by either assembling lighter nuclei into heavier ones (fusion) or by breaking heavier nuclei into lighter nuclei (fission).
Þ Homework (check the above statement)
Fission and fusion can yield energy
- Trying to understand this curve led to semiempirical mass formula.
B/A
- We would expect B
µ
Þ nucleon interact only with their closest neighbors.
Þ short distance effect ( for the nuclear force )
- B = av
A is an overestimate because nucleons on the surface don't contribute the
same as inner nucleons, so we have to subtract a term
proportional to Surface Area ~ (why
So
B =
We also need a term µ Coulomb interaction or Z (Z-1), why ? (since each proton repel all others)
So
B =
- We also need a term that favors nuclei along the line Z=N or Z = A/2
- -
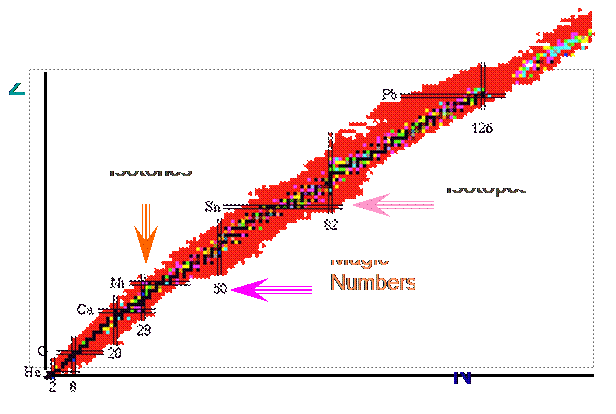
- From observing nuclei in nature they found that there are only 4 stable nuclei with odd N and Z
Þ There must be a pairing term d ( other forms exist )
Where
ì ap A-3/4 for even N and Z ď d = í 0 for odd A (even Z and odd N or vice versa) ď î - ap A-3/4 for odd N and Z
-
M(Z, A) = Z mH + N mn – B (Z, A)/ c2 - For constant A This is an equation of a parabola ( See Fig 3.18) - We can find the minimum by differentiating ¶M / ¶Z = 0 |