An ideal gas, initially occupies a volume of 0.380 m**3
at a pressure of 2.04*10**5 Pa, expands isothermally to
a pressure of 1.01*10**5 Pa.
Calculate the work done by the gas.
a 54.5 kJ
b 321 kJ
c 27.0 kJ
d 539 kJ
e 32.1 kJ
==============================================
Question 2
For an ideal gas, Which of the following statements are CORRECT:
1. Cp – Cv = R/2.
2. In an isothermal process, the internal energy of the
system does not change.
3. In an adiabatic process, no heat enters or leaves the system.
4. In a constant volume process, the work done by the system is
positive.
a 1, 2 and 4.
b 2 and 3.
c 3 and 4.
d 1 and 3.
e 2 and 4.
==============================================
Question 3
For a given temperature increase, a certain amount of
an ideal gas requires 80 J of heat when heated at
constant volume and 150 J of heat when heated at
constant pressure. How much work is done by the gas
in the second situation ?
a +70 J
b -80 J
c zero
d -70 J
e +80 J
==============================================
Question 4
Five moles, of an ideal monatomic gas, expand at constant
pressure of 1.0*10**2 Pascal from a volume of 1.0 m**3 to
to a volume of 3.0 m**3. What is the change in the internal
energy of the system?
a 300 J.
b 1000 J.
c 100 J.
d 600 J.
e 500 J.
==============================================
Question 5
An ideal gas occupies a volume of 12 L at 20 degrees-C
and a pressure of 1.0 atm. Its temperature is now raised
to 100 degrees-C and its pressure increases to 3.0 atm.
The new volume is:
a 25 L.
b 5.1 L.
c 0.20 L.
d 14 L.
e 21 L.
==============================================
Question 6
Two moles of helium (monatomic) gas are heated from 100
degrees Celsius to 250 degrees Celsius. How much heat is
transferred to the gas if the process is done at constant
pressure?
a 3.11 kJ.
b 8.52 kJ.
c 2.63 kJ.
d 1.51 kJ.
e 6.23 kJ.
==============================================
Question 7
An ideal gas containing 5.00 moles expands isothermally
at 127 degrees-C to four times its initial volume.
Find the heat flow during this expansion.
a 7.32 kJ out of the system
b 34.5 kJ out of the system
c 7.32 kJ into the system
d 23.0 kJ into the system
e 23.0 kJ out of the system
==============================================
Question 8
In an adiabatic process, the temperature of one mole of an
ideal monatomic gas is decreased from 500 K to 400 K.
What is the work done during the process in calories?
a 500
b 400
c 100
d 300
e 200
==============================================
Question 9
Two moles of a monatomic ideal gas is compressed at a constant
pressure of 1.5 atm from a volume of 70 liters to 35 liters.
Calculate the change in internal energy of the gas.
a - 0.87*10**4 J
b - 1.3*10**4 J
c - 1.9*10**4 J
d - 3.5*10**4 J
e 2.4*10**4 J
==============================================
Question 10
Which of the following statements is CORRECT ?
a For a given medium, the frequency of a wave is
inversely proportional to wavelength.
b The internal energy of an ideal gas depends on the
temperature and pressure only.
c A standing wave must be transverse.
d The rms speed of gas molecules decreases in
an isotherml process.
e Heat is a temperature difference.
==============================================
Question 11
An ideal gas undergoes an isothermal process starting with a
pressure of 2*10**5 Pa and a volume of 6 cm3. Which of the
following might be the pressure and volume of the final state?
a 6*10**5 Pa and 2 cm**3
b 4*10**5 Pa and 4 cm**3
c 8*10**5 Pa and 2 cm**3
d 1*10**5 Pa and 10 cm**3
e 3*10**5 Pa and 6 cm**3
==============================================
Question 12
One mole of an ideal monatomic gas is taken through
the cycle of figure 5.
Calculate the work done in a complete cycle.
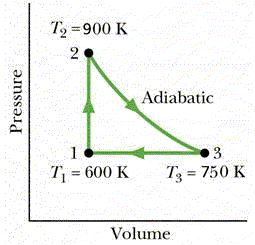
a + 325 J
b - 525 J
c + 895 J
d zero
e + 623 J
==============================================
Question 13
One mole of an ideal monatomic gas is taken from A to C
along the diagonal path in figure 5. What is the change
in the internal energy of the gas in this process ?
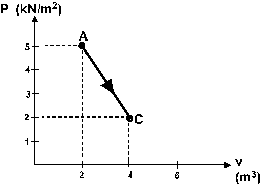
a - 5.0 kJ
b ZERO
c - 3.0 kJ
d + 5.0 kJ
e + 3.0 kJ
==============================================
Question 14
Initially, an ideal monatomic gas containing 10.0 moles
occupies a volume of 30.0 L at a pressure of 5.00 atm.
It is then compressed adiabatically to a final volume of
12.0 L. What is the final temperature of the gas ?
a 844 K
b 457 K
c 157 K
d 420 K
e 336 K
==============================================
Question 15
One mole of an ideal monatomic gas is taken through the cyclic
process shown in the figure. Calculate the net heat (lost or
gained) by the gas during one complete cycle.

a 4*Po*Vo Joule (lost)
b 2*Po*Vo Joule (gained)
c 3*Po*Vo Joule (lost)
d 4*Po*Vo Joule (gained)
e 2*Po*Vo Joule (lost)
==============================================
Question 16
An ideal diatomic gas, initially at a pressure Pi = 1.0 atm
and volume Vi, is allowed to expand isothermally until it’s
volume doubles. The gas is then compressed adiabatically
until it reaches its original volume. The final pressure of
the gas will be:
a 1.3 atm.
b 0.5 atm.
c 1.7 atm.
d 2.0 atm.
e 0.4 atm.
==============================================
Question 17
Two moles of nitrogen are in a 3-liter container at a
pressure of 5.0*10**6 Pa. Find the average translational
kinetic energy of a molecule.
a 1.0*10**(-24) J.
b 1.1*10**(-23) J.
c 3.6*10**(-20) J.
d 1.9*10**(-20) J.
e 7.1*10**(-22) J.
==============================================
Question 18
The figure shows 5 paths traversed by a gas on a P-V diagram.
For which of the 5 paths is the change in internal energy
the greatest?

a 5
b 3
c 4
d 1 and 2
e 5 and 4
==============================================
Answers
1 a
2 b
3 a
4 a
5 b
6 e
7 d
8 d
9 b
10 a
11 a
12 e
13 c
14 e
15 c
16 a
17 d
18 a