Prepared
by Dr. A. Mekki
Summary of chapter
19
1.
If
two bodies are in thermal equilibrium
with each other they must have the same
temperature.
2.
The Zeroth-law of thermodynamics states that if two bodies A and B are
separately in thermal equilibrium with a third body, C,
then A and B are in thermal equilibrium
with each other when placed in thermal contact.
3.
Change
of temperature scale:
TC
= TK 273
TF
= 9/5 TC + 32
TK
= 5/9 TF + 255
Important: ![]() and
and
![]()
4.
When
a substance is heated, it generally expands. The change in length, DL is related to the change in
temperature DT
and the
proportionality constant is called a (coefficient of linear expansion)
5.
Expansion and contraction of a solids
Ø In one dimension
The change in length is given
by ![]() and
and
The final length is ![]()
Li is the initial length,
Lf is the final length, and a is the coefficient of
linear expansion
Ø In two dimensions
The change in the area is
given by ![]() and
and
The final area is ![]()
Ø In three dimensions
The change in
the volume is ![]() and
and
The final volume
is
![]() This equation is valid for
solids and liquids.
This equation is valid for
solids and liquids.
Coefficient of
volume expansion ![]() .
.
6.
The
heat gained or lost by a substance is given by:
(i)
If there is a change in temperature and there is no change in the phase
![]()
c is the
specific heat of the substance and DT = Tf - Ti
(ii)
If there is
a change in phase and the temperature of the system remains the same
![]()
L is called the heat of transformation.
If there is fusion (solid liquid), then we use Lf, if there is vaporization
(liquid gas), then we use Lv.
7.
This
section is related to GASES ONLY
A gas may exchange energy with
the surroundings through work.
The work done on or
by a gas as it expands or contract from Vi to Vf
is
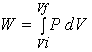
 The work can also be
calculated from a PV diagram.
The work can also be
calculated from a PV diagram.
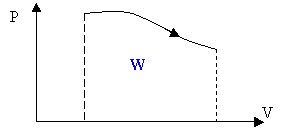
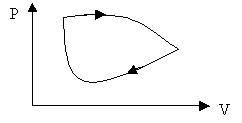
The work is the area under the curve in a PV diagram as shown in the above figure.
For a cyclic process the work is the area enclosed by the cycle.
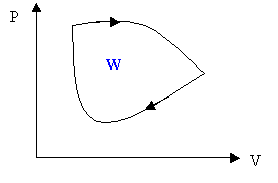
8.
The first law of thermodynamics is the law of conservation of energy and given by
![]()
where DEint is the internal energy of the gas.
Ø Q > 0 if the gas absorbs (gains) heat
Ø Q < 0 if the gas expels (lose) heat
Ø
W > 0 if the gas
does work
Ø W < 0 if external work is done on the gas
9.
Special cases of the first
law of thermodynamics
Ø Adiabatic process: Q
= 0 and DEint = - W
Ø Constant volume process: W = 0 and DEint = Q
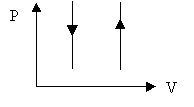
Ø Cyclic process: DEint = 0, Q = W
Ø Free expansion: DEint
= Q = W = 0
10.
Heat can be transferred
between a system and the environment in three ways; by conduction, by convection, and
by radiation.
Ø In the case of transfer of heat by conduction, the rate of
heat flow is given by:
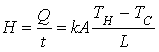 (Watts)
(Watts)
k is the thermal conductivity of the material through
which heat is conducted.
Ø Radiation is heat transfer through the emission of
electromagnetic energy. The power of the radiating heat source is given by:
![]() (Watts)
(Watts)
s = 5.6703 10-8
W/m2K is Stefan-Boltzman constant.
e is the emissivity of the object and A is
its surface area of the heat source.
T
is the temperature in Kelvin.