| Objective |
To demonstrate pitting on the surface of different types of steels |
|
| Materials | Coupon of stainless steel, low carbon steel, high carbon steel | |
|
Electrolyte: |
||
|
|
||
| Procedure |
Immerse the steel coupons in the above solution for one week in Plexiglas containers. Then remove them from the solution and clean with acetone and dry them before observation under Scanning Electron microscope. |
|
|
|
||
| Observations |
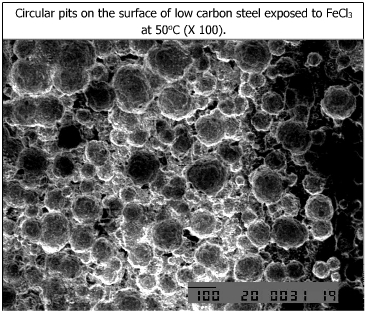
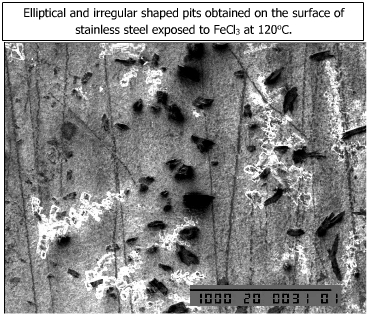
The microstructures of the corroded specimens are shown below.
|
|
|
Circular pits are observed on low carbon steel. |
|
|
|
Adjacent figure shows the pits at a higher magnification. |
|
|
|
A pit covered by corrosion product in a circular shape is shown in this figure. |
|
|
|
Circular pits formed on high carbon steels are shown here. |
|
|
|
One single magnified pit is shown in the adjacent figure. |
|
|
| Elliptical and irregular shaped pits are shown in the adjacent figure. |
|
|
| Conclusions | Pitting is a very severe form of corrosion mostly caused by the ingress of chloride ion which destroys the passive film on the steel surface. | |