| 5. Cathodic Protection | |
|
5.3 Cathodic Protection Systems [1/8] |
|
There are two types of cathodic protection systems
Cathodic protection system using Galvanic anodes
Cathodic protection systems using impressed current anodes.
1 Cathodic Protection Using Galvanic Anodes (sacrificial anodes)
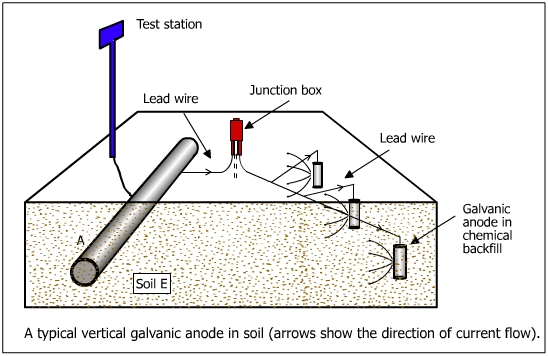
In the galvanic anode system, the cathodic protection current is derived from a corroding metal, known as the anode. Active metals corrode rapidly when put in an electrolyte (eg. soil). For instance magnesium is very corrosive (E = +2.37 V) and it would corrode rapidly when exposed to soil. Similarly, aluminum and zinc would corrode and release electrons. These materials are allowed to corrode to derive the current required for cathodic protection . The above three materials are used as galvanic anodes for cathodic protection of underground structures. The galvanic system is illustrated in figure below.

The positive current flows from the sacrificial anode to the pipe through the soil. A number of magnesium or zinc anodes are connected to the pipeline by an insulated cable. The connections are made above ground in a test post so that the readings of potential and currents are taken. The junction box in the insulated wire connecting the anodes to the pipe facilitates the measuring of pipe to soil potential on the current supplied by the anode.
|
|