| 4. Forms of Corrosion | |
|
4.11 Crevice Corrosion [2/2] |
|
Mechanism
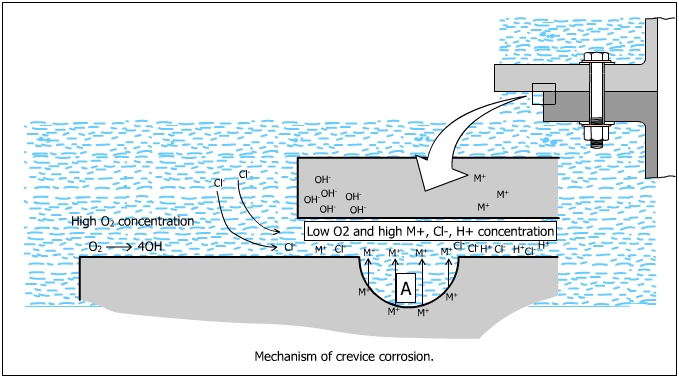
Consider bolted metal plates exposed to seawater as shown in the figure below. The small crevice (A) inside the assembly forms the anode and the outside area, the cathode.
At the anodic areas, as usual oxidation would occur inside the
crevices which form the anodes. M ![]() Mz+ + ze
Mz+ + ze
The oxygen inside the crevice is depleted due to the consumption in the cathodic reaction. The phenomenon inside the crevice makes the surface area inside the crevice a small anode compared to the outside of a crevice. This is virtually the formation of a differential aeration cell. The formation of differential aeration cell accelerates the metal dissolution inside the crevice.
At the cathodic areas, reduction of oxygen takes place
O2 + 2H2O + 4e![]() 4OH-
4OH-
Inside the Crevice:
The amount of oxygen inside the crevice is depleted due to its consumption in the cathodic reactions such as the reaction utilizing the Cl- ions from the electrolyte. The metal in the crevice, however, continues to corrode and an excess of metal ions and Mz+ are accumulated. These positive ions attract the negative Cl- ions and a metal chloride (MCl) is formed, which reacts with water for form a metal hydroxide (MOH) which leaves a free acid (M+Cl-).
M+Cl- +
HOH![]() MOH + H+Cl-
MOH + H+Cl-
The production of free acid causes metal dissolution and accelerates the anodic reaction. Each ion which dissolves, releases an electron which flows through the metallic particle and combines with oxygen to form hydroxide ions, outside the crevice. The outside of the crevice is protected by the cathodic protection and it does not corrode.
Propagation of Crevice Corrosion
As more and more anodic dissolute proceeds, more Cl- ions are attracted, more hydrolysis occurs and crevice corrosion proceeds unchecked.
Classical examples
Corrosion under thermal insulation
Denting in nuclear generators
Blockage of heat exchanger tubes
Deposits of dirt and mud in vehicle
Prevention
Eliminate crevices during design. Butt welds are preferred over rivets or bolts. Eliminate crevice through welding, caulking and use of gaskets.
Select alloys more resistant to corrosion such as 316 SS. 9-10 copper-nickel and aluminum bronze alloys for seawater service. Use Cr, Ni, Mo alloyed steels.
Use replaceable parts if possible to avoid plant shut down.
Minimize regions of aggressive ions such as chloride.
Use cathodic and anodic inhibitors. The inhibitors have an insulating effect and block the flow of electrons. Care has to be taken in adding anodic inhibitors in sufficient concentration.
|
|