| 4. Forms of Corrosion | |
|
4.5 Intergranular Corrosion [1/3] |
|
Intergranular Corrosion (IGC)
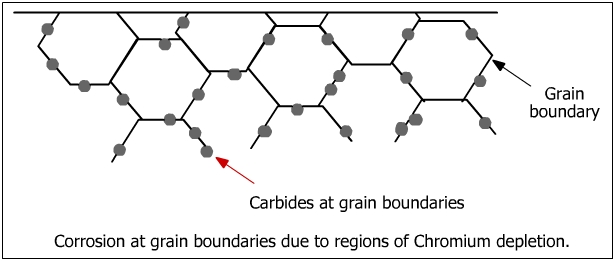
The microstructure of metals and alloys is made of grains separated by grain boundaries (see figure below). Intergranular attack is a localized attack adjacent to the grain boundaries in which the bulk of the grains are not affected.
Materials susceptible to intergranular corrosion:
Steels, brasses, bronzes and aluminum alloys are all susceptible to intergranular corrosion. The grains in all crystalline materials are separated by grain boundaries which are susceptible to IGC. Because of the importance of steels, the presentation here is confined to steels.
Sensitization of steels:
When steels are exposed to a certain range of temperature such as during welding or slow cooling, they become susceptible to IGC and are referred to as sensitized steels. The process of damaging thermal exposure is called sensitization. When steels such as austenite steels are exposed to sensitization temperature zone (560 - 580 oC), they become susceptible to IGC.
Grain boundary corrosion visible in 316 L Stainless steel etched in acidified CuCl2.
Factors affecting sensitization of steels:
| 1. Carbon content | Higher amounts of carbon cause
sensitization in a shorter period than small amounts of carbon.
Example: A steel containing 0.06% C becomes sensitized at 600 C in less than 1 hour, whereas a steel containing 0.03 % C takes more than 25 hours to sensitize. |
| 2. Nickel content | The susceptibility increases with increased nickel content. |
| 3. Molybdenum | The susceptibility decreases with increase in molybdenum. |
| 4. Titanium and Columbum | These elements combined with carbon in preference to chromuim and do not allow the combination of carbon with chromium which is primarily responsible for ICG. |
|
|