

| 4. Forms of Corrosion | |
|
4.3 Galvanic Corrosion [1/4] |
|
Galvanic Corrosion
It is the corrosion caused by joining of two different metals exposed to an electrolyte. The factor responsible for corrosion is the difference of potential between the two metals which is the driving force for galvanic corrosion.
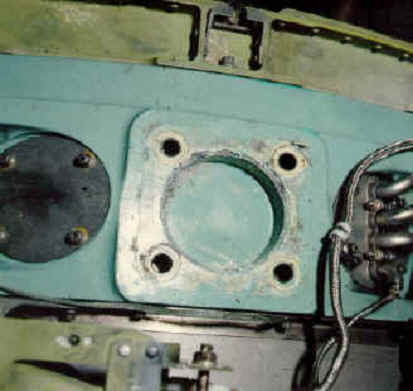
Some typical cases of Galvanic Corrosion are shown below:
Other Examples
1. Use of lead sheathing on hulls to protect the wood from worms.
2. Joining of old pipes with a new pipe.
3. Brass fittings in steel pipes.
4. Joining of steel main pipes with the copper outlets of a domestic water heater.
5. Painting the corroded area of the metal and leaving the uncorroded area.
6. Galvanic corrosion inside horizontal stabiliser in aircrafts.
7. Galvanic corrosion of Statue of Liberty.
|
|