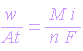
| 3. Faraday's Laws and Rate of Corrosion | |
|
3.3 Corrosion Penetration Rate |
|
The rate of corrosion can be expressed in terms of current density as
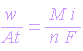
Penetration unit time can be obtained by dividing the above equation by density of the alloy. The following equation can be used conveniently to express corrosion rate r
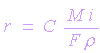
| where |
| r = density |
| i = current density (A/cm2) |
| M = atomic weight (g/mole) |
| n = number of electrons involved |
| C = constant which includes F and any other conversion factor for units, for instance, C = 0.129 when corrosion rate is in mpy, 3.27 when in mm/yr and 0.00327 when unit are in mm/y-1 |
For instance the above relationship can be used to establish the equivalent of corrosion current of μA/cm2 with rate of corrosion for iron in mpy as shown below
1 mA/cm2 = 0.129 (55.8 x 1)/(2 x 7.86) = 0.457 mpy
Thus, for iron 1 μA/cm2 of current is equivalent to a corrosion rate of 0.46 mpy for iron. The equation can be extended to establish the equivalent in other units also.
The above example shows the correspondence between penetration rate and current density for a metal. A similar correspondence between the penetration rate and current density for alloys can be established. However, it would require the determination of equivalent weight (M/n) for the alloy.
Following is the relationship which is used to determine the equivalent weight of an alloy:
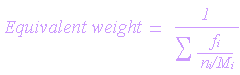
| where |
| fi = mean fraction of an element present in the alloy |
| n1 = electron exchanged |
| M1= atomic mass |
|
|