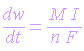
| 3. Faraday's Laws and Rate of Corrosion | |
|
3.2 Determination of Corrosion Rates |
|
Corrosion rate has dimensions of mass/time (g/s or g/year or kg/s)
In terms of loss of weight of a metal with time
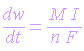
The rate of corrosion is proportional to the current passed to the molar mass. Dividing the above equation by exposed area (A) of the metal in the alloy
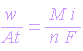
But
|
(current density) |
Therefore,
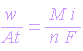
The above equation has been successfully used to determine the rates of corrosion. A very useful unit for representing the corrosion rate is milligrams per decimeter square per day (mg.dm-2.day-1) or mdd. Other units are millimeter per year (mmy-1) and mils per year (mpy).
|
|