| 2. Understanding Corrosion | |
|
2.8 Concentration Polarization [2/2] |
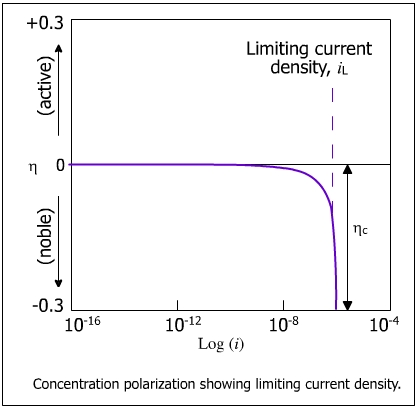
The concentration polarization at the anode can be ignored because of the abundance of positive ions at the electrode/electrolyte interface. As the rate of discharge of H+ ions overrides the rate of diffusion of the ions from the bulk solution, the concentration of H+ ions continue to decrease until it reaches zero at the electrode/electrolyte interface. The current at this stage reaches a limiting value of the current density (iL) and becomes independent of potential. This behavior is shown in the figure below.
Concentration polarization is expressed as
hconc = (2.3RT/nF) Log (i - i/iL)
| i = applied current density, mA/cm2 |
| iL = limiting current density for a cathodic process |
The limiting current is defined as
iL = D n Z F C / d (1 - t)
| D = diffusion coefficient, cm2/s |
| nZ = number of electrons |
| F = Faradays, Couloumbs/mole |
| d = thickness of diffusion layer, cm |
| t = transfer number (0.1-0.9) from the electrode surface |
| C = concentration of diffusing species, moles/litre |
It is to be remembered that at low cathodic polarization, the reduction process is activation controlled whereas at high cathodic polarization it is concentration controlled. When the reduction current reaches iL activation polarization is taken over by concentration polarization. The total polarization is the total of activation polarization and concentration polarization.
