-
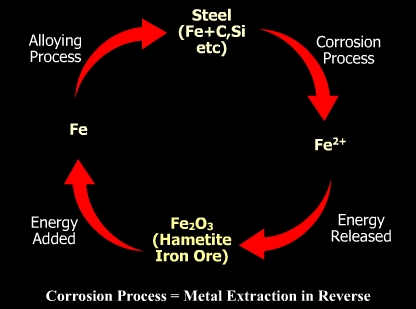
All metals are extracted from their ores. For instance, aluminum is extracted from bauxite by electrolysis and iron is obtained by melting of its ore hametite (Fe2O3).
-
Metals store heat as potential energy during the melting and refining process and release this energy during the corrosion process after reacting with the environment. The starting material for iron and steel making is Fe2O3 and the corrosion product (rust) has the same chemical composition.
-
Metals and alloys return to their original state after corrosion. Different amounts of energy are expended to reduce the oxides to free metals, depending on the ore type and the refining process.
-
The energy stored during the refining process is converted to kinetic energy during the corrosion process which returns the metal or alloy to its native state (ore).
-
The energy stored during melting and released during corrosion supplies the driving potential for the corrosion process to take place.
-
A corrosion cycle is shown below.
Example: Metals such as Mg, Al, Zn, Fe which require larger amounts of energy for refining are more susceptible to corrosion than metals which require lesser amounts for refining such as gold, silver, platinum, etc.