| Home | C.V. | Teaching | Research | Useful Links | Contact me |
Induced laser
Laser is an acronym for light amplification by stimulated emission of radiation. Lasers are devices that amplify light and produce coherent light beams, ranging from infrared to ultraviolet. A light beam is coherent when its waves, or photons, propagate in step with one another. Laser light, therefore, can be made extremely intense, highly directional, and very pure in color (frequency). Laser devices now extend into the X-ray frequency range.

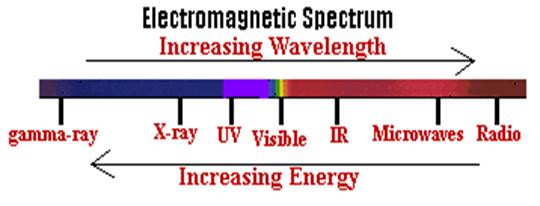
Fig 1. Regions of Electromagnetic Spectra
1. PRINCIPLES OF OPERATION
Lasers harness atoms to store and emit light in a coherent fashion. The electrons in the atoms of a laser medium are first pumped, or energized, to an excited state by an energy source. They are then “stimulated” by external photons to emit the stored energy in the form of photons, a process known as stimulated emission. The photons emitted have a frequency characteristic of the atoms and travel in step with the stimulating photons. These photons in turn impinge on other excited atoms to release more photons. Light amplification is achieved as the photons move back and forth between two parallel mirrors, triggering further stimulated emissions. The intense, directional, and monochromatic laser light finally leaves through one of the mirrors, which is only partially silvered.
Stimulated emission, the underlying process for laser action, was first proposed by Albert Einstein in 1917. The working principles of lasers were outlined by the American physicists Arthur Leonard Schawlow and Charles Hard Townes in their 1958 patent application. The patent was granted, but was later challenged by the American physicist and engineer Gordon Gould. In 1960 the American physicist Theodore Maiman observed the first laser action in solid ruby. A year later a helium-neon gas laser was built by the Iranian-born American physicist Ali Javan. Then in 1966 a liquid laser was constructed by the American physicist Peter Sorokin. The U.S. Patent Office court in 1977 affirmed one of Gould's claims over the working principles of the laser.
2. TYPES OF LASERS1
Based on the laser medium used, lasers are generally classified as solid state, gas, semiconductor, liquid or free electron lasers.
2.1 Solid-State Lasers
The most common solid laser media are rods of ruby crystals and neodymium-doped glasses and crystals. The ends of the rod are fashioned into two parallel surfaces coated with a highly reflecting nonmetallic film. Solid-state lasers offer the highest power output. They are usually operated in a pulsed manner to generate a burst of light over a short time. Bursts as short as 12 × 10-15 sec have been achieved, useful in studying physical phenomena of very brief duration. Pumping is achieved with light from xenon flash tubes, arc lamps, or metal-vapor lamps. The frequency range has been expanded from infrared (IR) to ultraviolet (UV) by multiplying the original laser frequency with crystal-like potassium dihydrogen phosphate, and X-ray wavelengths have been achieved by aiming laser beams at an yttrium target.
2.2 Gas Lasers
The laser medium of a gas laser can be a pure gas, a mixture of gases, or even metal vapor and is usually contained in a cylindrical glass or quartz tube. Two mirrors are located outside the ends of the tube to form the laser cavity. Gas lasers are pumped by ultraviolet light, electron beams, electric current, or chemical reactions. The helium-neon laser is known for its high frequency stability, color purity, and minimal beam spread. Carbon dioxide lasers are very efficient, and consequently they are the most powerful continuous wave (CW) lasers.
2.3 Semiconductor Lasers
The most compact of lasers, the semiconductor laser usually consists of a junction between layers of semiconductors with different electrical conducting properties. The laser cavity is confined to the junction region by means of two reflective boundaries. Gallium arsenide is the most common semiconductor used. Semiconductor lasers are pumped by the direct application of electrical current across the junction, and they can be operated in the CW mode with better than 50 percent efficiency. A method that permits even more-efficient use of energy has been devised. It involves mounting tiny lasers vertically in such circuits, to a density of more than a million per square centimeter. Common uses for semiconductor lasers include compact audio digital disk (CD) players and laser printers.
2.4 Liquid Lasers
The most common liquid laser media are inorganic dyes contained in glass vessels. They are pumped by intense flash lamps in a pulse mode or by a gas laser in the CW mode. Tunable dye lasers are a type for which frequency can be adjusted with the help of a prism inside the laser cavity.
2.5 Free Electron Lasers
Lasers using electrons unattached to atoms and pumped to lasing capacity by an array of magnets were first developed in 1977 and are now becoming important research instruments. They are tunable, as are dye lasers, and, in theory, a small number could cover the entire spectrum from infrared to X rays. Free electron lasers should also become capable of producing very high-power radiation that is currently too expensive to produce [1].
|
Type |
Wavelength |
Efficiency |
Power levels available (W) |
|
|
Pulsed |
CW |
|||
|
CO2 |
10.6 |
0.01 - 0.02 |
> 2 ´ 1013 |
> 105 |
|
CO |
5 |
0.4 |
> 109 |
> 100 |
|
Holmium |
2.06 |
0.03 (lamp) |
> 107 |
30 |
|
Iodine |
1.315 |
0.003 |
> 1012 |
- |
|
Nd-glass, |
1.06 |
0.001 - 0.06 (lamp) |
~ 1014 |
1 - 103 |
|
* Color center |
1 - 4 |
10-3 |
> 106 |
1 |
|
* Vibronic |
0.7 - 0.9 |
> 0.1 hp |
106 |
1 - 5 |
|
Ruby |
0.6943 |
< 10-3 |
1010 |
1 |
|
He-Ne |
0.6328 |
10-4 |
- |
1 - 50 ´ 10-3 |
|
* Argon ion |
0.45 - 0.60 |
10-3 |
5 ´ 104 |
1 - 20 |
|
* OPO |
0.4 - 9.0 |
> 0.1 ´ hp |
106 |
1 - 5 |
|
N2 |
0.3371 |
0.001 - 0.05 |
105 - 106 |
- |
|
* Dye |
0.3 - 1.1 |
10-3 |
> 106 |
140 |
|
Kr - F |
0.26 |
0.08 |
> 109 |
500 |
|
Xenon |
0.175 |
0.02 |
> 108 |
- |
* = Tunable sources, hp=pump laser efficiency
YAG stands for Yttrium Aluminum Garnet and OPO for Optical Parametric
Oscillator; η p is pump laser efficiency.
3. DYE LASER 2,3
The unique characteristic of a dye laser is that it is tunable - the user can select a desirable output frequency within a certain range characteristic of the dye.
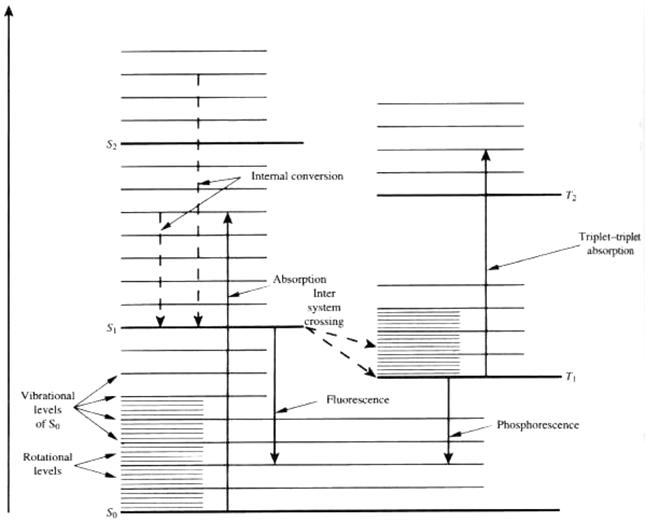
Fig 2. Jablonski Diagram of Energy Levels
As the CO2 laser, the lasing/amplifying medium of the Dye laser is a molecule. With each electronic energy level of the molecule are associated vibrational and rotational energy levels. These dye molecules are hydrocarbon molecules with carbon-carbon double bonds and sequences of alternating single and double bonds. All of these molecules have the hexagonal carbon ring structures. These rings have associated loosely bound electrons that can move from different nuclei within the plane of the ring. It is these electrons that provide the energy level structures of the dye.
At any time, there is always an even number of electrons in the ground state. These are grouped in pairs: one with its spin momentum vector pointed up and the other with its vector pointed down. Therefore, the total angular momentum is zero (they are in the ground state).
A single electron from one pair can be excited into a series of states. In the following diagram, S1 and S2 are singlet states, T1 and T2 are triplet states and S0 is the ground state:
The difference between these two 'genres' of states is that in a singlet state, the excited electron has moved to a higher energy level, but retains its original spin. Therefore, its total angular momentum remains 0. In a triplet state, when excited electrons transition to a higher energy state, the spin flips. Thus there is a net angular momentum for these states.
Each singlet and triplet state has, as previously mentioned, associated rotational and vibrational energy states. Vibrational and rotational levels are close together relative to the electronic-level spacing. The vibrational levels are on the order of 1200-1700 cm-1, and the rotational levels are even smaller by two orders of magnitude. At room temperature, most dye molecules are in the vibrational state of So. If light of appropriate energy is incident on these molecules, excitation to a higher singlet state will probably occur.
The S0 to S1 transition covers a broad range of frequencies due to the rotational-vibrational energy levels. Once excited to the S1 state, these molecules can de-excite to the lowest vibrational level of S1 in a transition which takes ~ a pico-second. This non-radiative decay is referred to as internal conversion. Transitions from this lowest vibrational level of S1 to the vibrational levels of S0 can occur spontaneously. This is called fluorescence. A further possibility is that molecules nonradiatively move from S1 to T1, or S2 to T2, etc. This process, intersystem crossing occurs relatively slowly relative to internal conversion, but up to 10 ns. Phosphorescence, an unlikely process as it involves electrons de-exciting and flipping their spin, is the spontaneous emission from T1 to S0.
The pumping process works like this:
1. Rapid excitation of a
vibrational-rotational level of S1
2. Internal conversion to the lowest vibrational level of S1
3. Laser emission to the vibrational-rotational levels of S0
sufficiently far above the ground state that their populations are low.
The system needs the S1 state to be heavily populated because of the broad fluorescence spectrum. Furthermore, intersystem crossing populates T1 and absorption can occur from the same wavelengths as emitted in the fluorescence. This loss must be minimized and is done so by adding substances such as oxygen or detergents to the dye. This can further be done by using a dye solution in which intersystem crossing is unlikely to occur. Using the Nd-YAG laser, however, the transfer to the triplet state can be ignored because of the short laser pulses. When the pump intensity is sufficient to achieve an upper level population density, the lower level is scarcely populated.
Dye molecules have a broad emission curve which is responsible for their tenability. This characteristic allows a range of emission wavelengths from 10-60Ĺ. To tune the laser, manipulate the cavity so that the gain of most frequencies outside of the desired frequency is less than the loss. Often this is accomplished by replacing on of the cavity's mirrors with a diffraction grating. Tuning the laser is done by rotating the grating as shown:
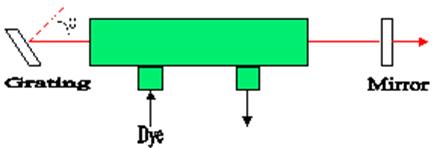
Fig 3. Diffraction Grating
Wavelengths satisfying the relation ![]() ,
where m = 1,2,... and d is the spacing between the lines on the grating are
amplified within the laser [2, 3].
,
where m = 1,2,... and d is the spacing between the lines on the grating are
amplified within the laser [2, 3].
4. LASER INSTRUMENTAL COMPONENTS 2, 4, 5
The Nd-YAG laser is an optically pumped solid-state laser that can produce very high-power emissions. This is a result of its lasing medium operating as a four-level system.
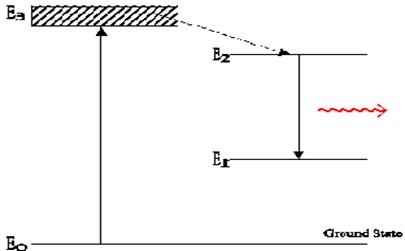
Fig 4. Laser Four-Level System
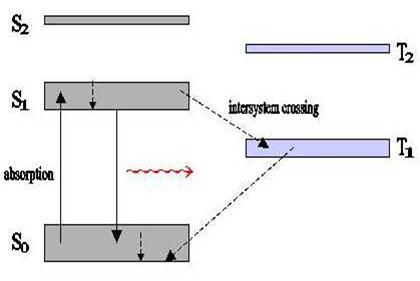
Fig 5. Laser Three-Level System
The lasing medium is the colorless, isotropic crystal Y2Al5O12 (Yttrium-Aluminum Garnet - YAG). When used in a laser, about 1% of the Yttrium is replaced by Neodymium. The energy levels of the Nd3+ ion are responsible for the fluorescent properties, i.e., active particles in the amplification process.
Population inversion results from shining light on this crystal. If the light is intense enough, atoms within the crystal that absorb this light transition from ground state into the absorption bands. This is often done with a flash lamp - often a quartz tube filled with a noble gas through which high energy stored in a capacitor is discharged, emitting in the blue and ultra-violet (see diagram below).
Atoms transition efficiently from their broad absorption bands (shown as the E3 level in the above diagram) to the upper energy (laser) levels. The radiative decays to the ground-state from these bands have long life-times, on the order of micro-seconds, as compared to the fast transitions to the upper energy levels (on the order of nano-seconds). Approximately 99% of the ions that are excited to the absorption band transfer to the upper energy levels. These levels are characterized by a relatively long lifetime, on the order of milli-seconds. Due to this long life-time, they de-excite almost solely due to spontaneous emission.
The Nd-YAG laser used in our lab uses a cylindrical crystal. The crystal forms the laser cavity and has reflective ends - one coated so that it is 100% reflective, and the other is either sufficiently reflective, or is coated to allow only part of the amplified light to pass - enough feed-back so that oscillation may occur. The following diagram may help visualize this apparatus [2, 4].
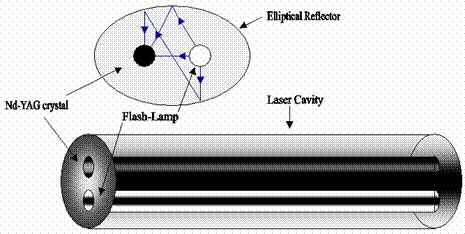
Fig 6. Nd-YAG Laser [Nd-YAG Cylinder]
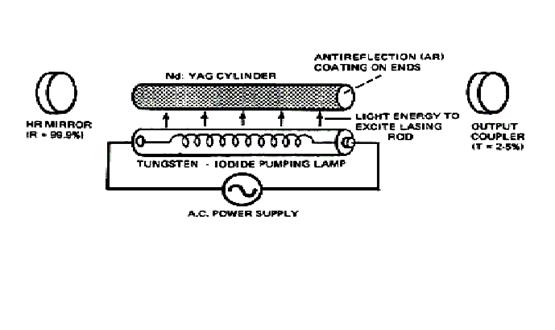
Fig 7. Nd-YAG Laser [Full Picture]
Lasers come in wide variety of forms, the processes that go on inside them differing greatly from one type of laser to another, but features that lasers have in common are:
 All
lasers contain an energized substance that can increase the intensity of light
passing through it. This substance is called the amplifying medium or,
sometimes, the gain medium, and it can be a solid, a liquid or a gas. Whatever
its physical form, the amplifying medium must contain atoms, molecules or ions,
a high proportion of which can store energy that is subsequently released as
light.
All
lasers contain an energized substance that can increase the intensity of light
passing through it. This substance is called the amplifying medium or,
sometimes, the gain medium, and it can be a solid, a liquid or a gas. Whatever
its physical form, the amplifying medium must contain atoms, molecules or ions,
a high proportion of which can store energy that is subsequently released as
light.
Fig 8. Amplifying Medium
In a neodymium YAG (Nd: YAG) laser, the amplifying medium is a rod of yttrium aluminum garnate (YAG) containing ions of the lanthanide metal neodymium (Nd). In a dye laser, it is a solution of a fluorescent dye in a solvent such as methanol. In a helium-neon laser, it is a mixture of the gases helium and neon. In a laser diode, it is a thin layer of semiconductor material sandwiched between other semiconductor layers. The factor by which the intensity of the light is increased by the amplifying medium is known as the gain. The gain is not a constant for a particular type of medium. Its magnitude depends upon the wavelength of the incoming light, the intensity of the incoming light, the length of the amplifying medium and also upon the extent to which the amplifying medium has been energized.
4.2 Energizing the Amplifying Medium
Increasing the intensity of a light beam that passes through an amplifying medium amounts to putting additional energy into the beam. This energy comes from the amplifying medium which must in turn have energy fed into it in some way. In laser terminology, the process of energizing the amplifying medium is known as "pumping".
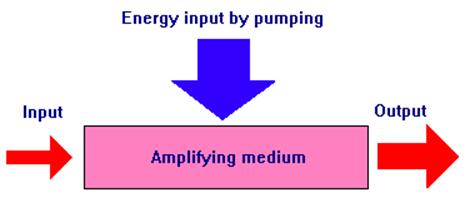
Fig 9. Energizing the amplifying medium
There are several ways of pumping an amplifying medium. When it is a solid, pumping is usually achieved by irradiating it with intense light. This light is absorbed by atoms or ions within the medium raising them into higher energy states. Xenon-filled flashtubes positioned as shown below are used as a simple source of pumping light. Passing a high voltage electric discharge through the flashtubes causes them to emit an intense flash of white light, some of which is absorbed by the amplifying medium. The assembly of flashtubes is enclosed within a polished metal reflector to concentrate as much light as possible on the amplifying medium. A laser that is pumped in this way will have a pulsed output.
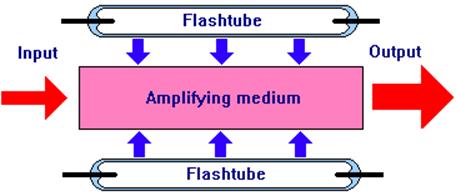
Fig 10. Pumping an Amplifying Medium
Pumping an amplifying medium by irradiating it with intense light is referred to as optical pumping. The source of pumping light can be another laser. Some types of laser that were originally pumped using xenon-filled flashtubes are now pumped by laser diodes.
Gaseous amplifying media have to be contained in some form of enclosure or tube and are often pumped by passing an electric discharge through the medium itself. The mechanism by which this elevates atoms or molecules in the gas to higher energy states depends upon the gas that is being excited and is often complex. In many gas lasers, the end windows of the laser tube are inclined at an angle and they are referred to as brewster windows. Brewster windows are able to transmit a beam that is polarized in the plane of the diagram without losses due to reflection. Such a laser would have an output beam that is polarized.
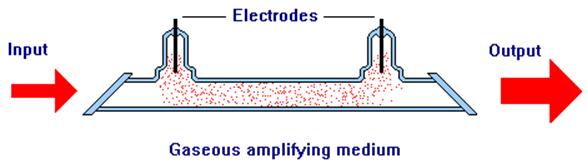
Fig 11. Gaseous Amplifying Medium
The diagram illustrates pumping by passing a discharge longitudinally through the gaseous amplifying medium but, in some cases, the discharge takes place transversely from one side of the medium to the other. Many lasers that are pumped by an electric discharge can produce either a pulsed output or a continuous output depending upon whether the discharge is pulsed or continuous.
Various other methods of pumping the amplifying medium in a laser are used. For example, laser diodes are pumped by passing an electric current across the junction where the two types of semiconductor within the diode come together.
4.3 Laser Oscillator
Pumped amplifying media could be used to increase the intensity of light at particular wavelengths and such amplifiers are often incorporated into laser systems. However, except in a few exceptional cases, light amplifiers would not be regarded as lasers. A laser consists of a pumped amplifying medium positioned between two mirrors. The purpose of the mirrors is to provide what is described as 'positive feedback'. This means simply that some of the light that emerges from the amplifying medium is reflected back into it for further amplification. Laser mirrors usually do not reflect all wavelengths or colors of light equally well - their reflectivity is matched to the wavelength or color at which the laser operates. In appearance, they do not look like ordinary mirrors and are transparent at some wavelengths. An amplifier with positive feedback is known as an oscillator.
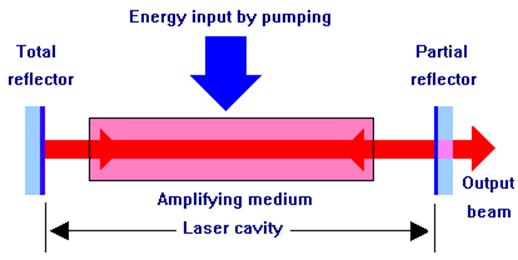
Fig 12. Laser oscillator
The space between the two mirrors is known as the laser cavity. The beam within the cavity undergoes multiple reflections between the mirrors and is amplified each time it passes through the amplifying medium. One of the mirrors reflects almost all of the light that falls upon it (total reflector in the above diagram). The other mirror reflects between 20% and 98% of the incident light depending upon the type of laser, the light that is not reflected being transmitted through the mirror. This transmitted portion constitutes the output beam of the laser.
The laser cavity has several important functions. Following pumping, spontaneous emission of light from excited atoms within the amplifying medium initiates the emission of low intensity light into the laser cavity. This light is increased in intensity by multiple passes through the amplifying medium so that it rapidly builds up into an intense beam. In the absence of cavity mirrors, this self-starting process, or oscillation, would not occur.
The cavity ensures that the divergence of the beam is small. Only light that travels in a direction closely parallel to the axis of the cavity can undergo multiple reflections at the mirrors and make multiple passes through the amplifying medium. More divergent rays execute a zig-zag path within the cavity and wander out of it.
The laser cavity also improves the spectral purity of the laser beam. Usually, the amplifying medium will amplify light within a narrow range of wavelengths. However, within this narrow range, only light of particular wavelengths can undergo repeated reflection up and down the cavity. The characteristics that a light beam within the cavity must possess in order to undergo repeated reflections define what is referred to as a cavity mode. Light which may still be amplified by the amplifying medium but which does not belong to one of these special modes of oscillation is rapidly attenuated and will not be present in the output beam. This behavior is similar to that of a vibrating guitar string in that a particular string will only vibrate at certain frequencies. In a similar way, an optical cavity will only sustain repeated reflections for particular well-defined wavelengths of light.
4.4 Absorption and Emission of Light by Atoms
So far, nothing has been written about how an amplifying medium amplifies light. Light can interact with individual atoms within an amplifying medium ("atoms" will be used to include molecules and ions). Atoms consist of a positively charged core (nucleus) which is surrounded by negatively charged electrons. According to the quantum mechanical description of an atom, the energy of an atomic electron can have only certain values and these are represented by energy levels. The electrons can be thought of as orbiting the nucleus, those with the largest energy orbiting at greater distances from the nuclear core. There are many energy levels that an electron within an atom can occupy, but here only two will consider.
4.4.1 Absorption and Spontaneous Emission
A photon of light is absorbed by an atom in which one of the outer electrons is initially in a low energy state denoted by 0. The energy of the atom is raised to the upper energy level, 1, and remains in this excited state for a period of time that is typically less than 10-6 second. It then spontaneously returns to the lower state, 0, with the emission of a photon of light. Absorption is referred to as a resonant process because the energy of the absorbed photon must be equal to the difference in energy between the levels 0 and 1. This means that only photons of a particular frequency (or wavelength) will be absorbed. Similarly, the photon emitted will have energy equal to the difference in energy between the two energy levels. These common processes of absorption and spontaneous emission cannot give rise to the amplification of light. The best that can be achieved is that for every photon absorbed, another is emitted.
4.4.5 Stimulated Emission
Stimulated emission is a very uncommon process in nature but it is central to the operation of lasers.
Above it was stated that an atom in a high energy, or excited, state can return to the lower state spontaneously. However, if a photon of light interacts with the excited atom, it can stimulate a return to the lower state. One photon interacting with an excited atom results in two photons being emitted. Furthermore, the two emitted photons are said to be in phase, i.e. thinking of them as waves, the crest of the wave associated with one photon occurs at the same time as on the wave associated with the other. This feature ensures that ther4e is a fixed phase relationship between light radiated from different atoms in the amplifying medium and results in the laser beam produced having the property of coherence. Stimulated emission is the process that can give rise to the amplification of light. As with absorption, it is a resonant process; the energy of the incoming photon of light must match the difference in energy between the two energy levels. Furthermore, if we consider a photon of light interacting with a single atom, stimulated emission is just as likely as absorption; which process occurs depends upon whether the atom is initially in the lower or the upper energy level. However, under most conditions, stimulated emission does not occur to a significant extent. The reason is that, under most conditions, that is, under conditions of thermal equilibrium, there will be far more atoms in the lower energy level, 0, than in the upper level, 1, so that absorption will be much more common than stimulated emission. If stimulated emission is to predominate, we must have more atoms in the higher energy state than in the lower one. This unusual condition is referred to as a population inversion and it is necessary to create a population inversion for laser action to occur.
4.5 Creating a Population Inversion
Finding substances in which a population inversion can be set up is central to the development of new kinds of laser. The first material used was synthetic ruby. Ruby is crystalline alumina (Al2O3) in which a small fraction of the Al3+ ions have been replaced by chromium ions, Cr3+. It is the chromium ions that give rise to the characteristic pink or red color of ruby and it is in these ions that a population inversion is set up in a ruby laser.

Fig 13. Population Inversion of Cr3+
In a ruby laser, a rod of ruby is irradiated with the intense flash of light from xenon-filled flashtubes. Light in the green and blue regions of the spectrum is absorbed by chromium ions, raising the energy of electrons of the ions from the ground state level to the broad F bands of levels. Electrons in the F bands rapidly undergo non-radiative transitions to the two metastable E levels. A non-radiative transition does not result in the emission of light; the energy released in the transition is dissipated as heat in the ruby crystal. The metastable levels are unusual in that they have a relatively long lifetime of about 4 milliseconds (4 x 10-3 s), the major decay process being a transition from the lower level to the ground state. This long lifetime allows a high proportion (more than a half) of the chromium ions to build up in the metastable levels so that a population inversion is set up between these levels and the ground state level. This population inversion is the condition required for stimulated emission to overcome absorption and so give rise to the amplification of light. In an assembly of chromium ions in which a population inversion has been set up, some will decay spontaneously to the ground state level emitting red light of wavelength 694.3 nm in the process. This light can then interact with other chromium ions that are in the meta stable levels causing them to emit light of the same wavelength by stimulated emission. As each stimulating photon leads to the emission of two photons, the intensity of the light emitted will build up quickly. This cascade process in which photons emitted from excited chromium ions cause stimulated emission from other excited ions is indicated below:
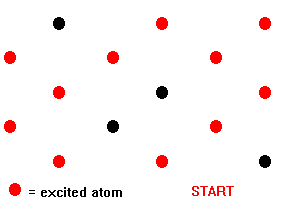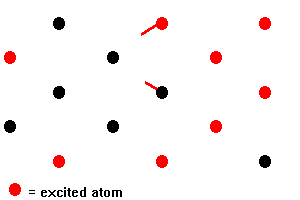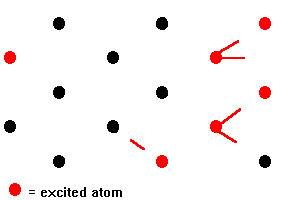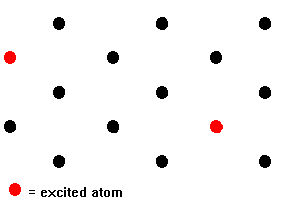
Fig 14. Stimulated Emissions
The ruby laser is often referred to as an example of a three-level system. More than three energy levels are actually involved but they can be put into three categories. These are; the lower level form which pumping takes place, the F levels into which the chromium ions are pumped, and the meta stable levels from which stimulated emission occurs. Other types of laser operate on a four level system and, in general, the mechanism of amplification differs for different lasing materials. However, in all cases, it is necessary to set up a population inversion so that stimulated emission occurs more often than absorption [5].
REFERENCES
1. Ching, Wu. Chu. Microsoft Encarta, 1997.
2. Davis, Christipherc. Lasers and Electro-optics. New York, 1996.
3. Milonni, Peter; Joseph Ebery. Lasers. New York, 1988.
4. Eastham, Derek. Atomic Physics of Lasers, 1986.
5. Web Science Resources (WSR), Laser Tutorial.